KEY POINTS
Current knowledge
• Infections are the most frequent cause of hospital admission in the first year after renal transplantation.
• The risk of infection during the post-transplant period is mainly determined by the nature and intensity of epidemiological exposure, the degree of immunosuppression, and the preventive measures used.
Contribution of the article to current knowledge
• In two third-level university hospitals in Cordoba city, the most frequent infections during the first year posttransplantation affected mainly the urinary tract and were caused by bacteria.
• Infection risk factors were age > 60 years, organ transplantation from a deceased donor, use of pigtail catheter for urinary tract drainage, and number of days in hospital after transplant.
End stage renal disease is an important cause of morbidity and mortality worldwide, and its incidence is growing constantly over the years1. Although renal transplantation is an effective treatment option with patients experiencing increased long-term survival, the concomitant implementation of immunosuppressive therapies has resulted in an increased risk of developing infections2,3. Infections are the main cause of hospital admission within 24 months after renal transplantation and account for prolonged hospital stays and increased healthcare costs. Furthermore, they represent the second leading cause of death in renal transplant recipients after cardiovascular disease4,5.
The risk of post-transplant infection is determined by several factors, including the nature and intensity of epidemiological exposure, the degree of immunosuppression, and the preventive measures used6,7. The frequency of infectious episodes varies during the first-year posttransplant. In the first month, infections are mainly caused by nosocomial (hospital-acquired) pathogens, surgical complications, and donor-derived infections. The period between one month and 6 months after transplantation is characterized by maximum immunosuppression, when there is greater susceptibility to develop opportunistic infections. By contrast, infections developing after 6 months post-transplant are usually caused by microorganisms which are common to the rest of the population8. It is important to understand the local epidemiology of the leading infectious conditions affecting renal transplant recipients in order to properly manage the patients.
The primary objective of this study was to determine the frequency of infectious disease in renal transplant recipients during a 1-year follow-up period after transplantation in our setting. The secondary objectives were: (i) to characterize the frequency and type of infections that occurred in renal transplant recipients during the early (0-1 month), intermediate (1-6 months), and late (6-12 months) post-transplant periods; (ii) to establish the frequency of reactivation of renal transplant recipient’s infections and opportunistic infections during the post-transplant period; (iii) to determine the frequency of donor-derived infections; and (iv) to assess the risk factors for developing infections during the post-transplant period.
Materials and methods
A retrospective study was carried out in two third-level university hospitals in Córdoba city, Argentina: Hospital Raúl Ángel Ferreyra and Hospital Privado Universitario de Córdoba. All patients aged 18 and older who received a single kidney transplant between January 2009 and December 2015, and who attended their follow-up visits at those hospitals were included. Patients under 18 years of age and those who had received another solid organ transplant during the same period were excluded.
All transplanted patients received immunosuppressive treatment and infection prophylaxis according to the Nephrology Department protocol. Patients with a high immunological risk received anti-thymocyte serum induction therapy (1.5 mg/kg/ day for 5 days), or basiliximab (2 doses of 20 mg) and human gamma globulin (2 doses of 2 g/kg) as an alternative regimen for subjects who were at a higher risk of developing serious infections and cancer. Patients with a high risk of delayed graft function and low immunological risk received basiliximab (same dosage). In addition, all patients received a methylprednisolone pulse therapy for 3 days. Calcineurin inhibitors (preferably tacrolimus), an anti-proliferative agent (preferably mycophenolate), and prednisone were used for maintenance therapy, except for a few special situations. All patients received antibiotic prophylaxis to prevent infection of the surgical site in the immediate post-surgery period, trimethoprim/sulfamethoxazole for one year, valganciclovir for 3 months (subjects who had received anti-thymocyte serum or subjects with negative CMV IgG results before transplant), or acyclovir for an indefinite period (all other patients), and nystatin during the first stage of the transplant. All patients who received an organ from donors with positive serology for syphilis (VDRL test) were given penicillin G benzathine (2 400 000 U). All subjects were tested for CMV, BK virus (BKV) and Chagas’ disease, with a monthly PCR determination up to 6 months. After transplantation, patients attended periodical follow-up controls which enabled the recording of significant events in their medical histories.
The medical history of each patient included in the study was reviewed, and demographics, comorbidities, transplantrelated information, and data about infections developed during the post-transplant period were collected. A distinction was made between standard criteria donors and expanded criteria donors (patients > 59 year or donors aged 50-59 who complied with at least two of the following three criteria: cerebrovascular accident as cause of death, history of hypertension and preablation serum creatinine level > 1.5 mg/dl)9. Patient follow-up was carried out until one-year post-transplant, until patients underwent a new solid organ transplantation, or until patient death or loss to follow-up, whichever occurred first.
The definitions used are found in the supplementary material.
The study was approved by the Hospital ethical review board.
Continuous variables were expressed as mean and standard deviation, and were compared with the Student’s t-test or the Mann-Whitney U test, according to their homogeneity. Categorical variables were expressed as frequency and percentage, and were compared using the chi-square test or the Fisher’s exact test, according to the expected frequencies. Statistical significance was defined as a p value < 0.05. Relative risk with 95% confidence interval was used to assess the link between individual risk factors and the development of infections during the post-transplant period. Subsequently, all significant variables with a p value < 0.05 in the univariate analysis were considered for a multivariate logistic regression analysis. Survival curves of patients who developed infections and patients who did not develop infections were compared, considering the period during which infections developed, using Kaplan-Meier survival curves and the log-rank test. The analyses were carried out using the Stata 14 statistical software (Stata-Corp. LP., College Station, TX, USA).
Results
Out of 488 patients who received a renal transplant during the study period, 113 (23.1%) were excluded. Of these, 76 (67.3%) were excluded because they had multiple solid organ transplantation, and 37 (32.7%) because they were under 18 years of age. A total of 375 patients were included, of whom 235 (62.7%) had at least one episode of infection during the follow-up period, and 140 had none.
Table 1 summarizes the epidemiological characteristics of the transplanted population together with donor, immunosuppression and transplant-related characteristics. The patients who had infection episodes were older (52 ± 14.9 vs. 46 ± 14.7 years old; p < 0.001) and had a history of diabetes mellitus more often than those who did not develop infections (18.7 vs. 10.7%; p = 0.04). Likewise, these patients also had a history of having more frequently received a kidney from a deceased donor (74 vs. 52.9%; p < 0.001) or from an expanded criteria donor (30 vs. 18.1%; p = 0.01), of having a greater delay in graft function (49.1 vs. 37.2%; p = 0.02), less use of pigtail catheter for urinary tract drainage (38.9 vs. 50.4%; p = 0.03), longer hospitalization time after transplant (11.6 ± 10.3 vs. 8.5 ± 4.4 days in hospital; p < 0.001), and more use of tacrolimus as maintenance immunosuppression (97 vs. 91.9%; p = 0.03). All these variables were considered as risk factors for infection after the transplant. The risk of infection increased by 38% in subjects over 60 years (RR = 1.38; 95% CI 1.20-1.60), 23% in diabetic patients (RR = 1.23; 95% CI 1.04-1.47), 46% in patients who received a kidney from a deceased donor (RR = 1.46; CI 95% = 1.19-1.78), 25% in patients who received an organ from an expanded criteria donor (RR = 1.25; 95% CI 1.07-1.45), 19% in patients who experienced a delay in graft function (RR = 1.19; 95% CI 1.02-1.39), and 65% in those patients who received tacrolimus (RR = 1.65; 95% CI 0.92-2.96). There were no significant differences in the presence of comorbidities such as heart failure, ischemic heart disease, chronic obstructive pulmonary disease, neurological, hepatic, rheumatic and neoplastic diseases between patients who had any episode of infection and those who did not have any during follow-up. There were also no differences in the modality and time of pre-transplant renal replacement therapy, invasive postsurgical procedures in the urinary tract, episodes of graft rejection, percentage of primary non-functioning transplants and type of immunosuppression used during induction and maintenance (except for tacrolimus). The median follow-up was 12 months and the loss to follow-up was 6.4%, with no differences found between the groups.

Table 1 Main features of renal recipient population according to the presence of infections within the irst-year post-transplant
In the multivariate analysis, infection risk factors adjusted for all other confounding variables were age > 60 years (adjusted odds ratio [OR] = 1 .92; 95% CI 1.05-3.49), organ transplantation from deceased donors (adjusted OR = 8.19; 95% CI 2.32-28.9), use of pigtail catheter for urinary tract drainage (adjusted OR = 4.06; 95% CI 1.27-12.9), and number of days in hospital after transplant (adjusted OR = 1.05; 95% CI 1.01 -1.11).
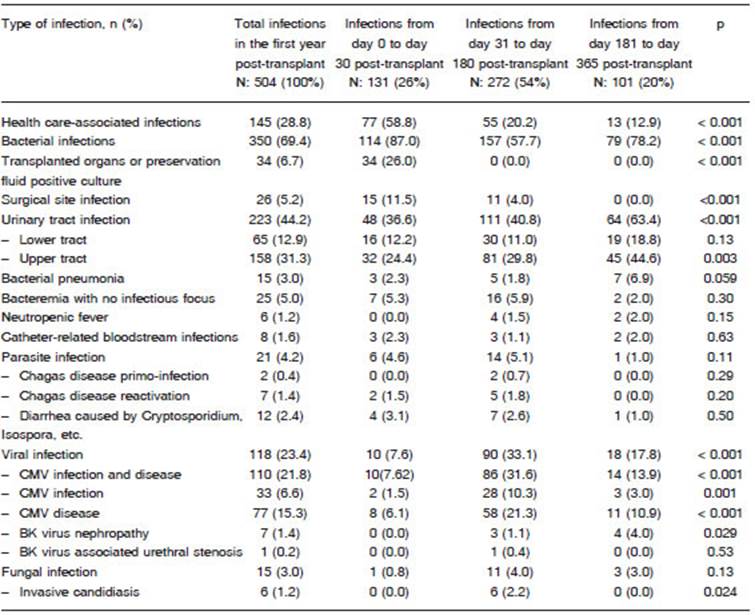
Five hundred and four infection episodes were reported, 131 (26%) of which occurred within the first 30 days after transplant, 272 (53.9%) occurred between 31 and 180 days after transplant, and 101 (20.1%) occurred between 181 and 365 days after transplant (Table 2).
According to their etiologic origin, the frequencies were bacterial 350 (69.4%), viral 118 (23.4%), parasitic 21 (4.2%), and fungal 15 (3%). Urinary tract infections (UTIs) were the most frequent infections (44.2%), followed by cytomegalovirus (CMV) infection and disease (23.4%), culture-positive preservation fluid or transplanted organ (6.7%), and surgical site infections (5.2%).
During the early period (0-30 days), most infections were caused by bacteria (87%), and a few infections were caused by viruses (7.6%). The most frequent infections during this period were UTIs (36.6%), culture-positive preservation fluid (26%) and surgical site infections (11.5%). During the intermediate period (31-180 days), there was an increase in the rate of viral infections (33.1%) and a relative decrease in the rate of bacterial infections (57.7%). The most frequent infections during this period were UTIs (40.8%) and CMV infection and disease (31.6%). During the late period (181-365 days), there was an increase in the rate of bacterial infections (78.2%) and a decrease in the rate of viral infections (17.8%). As it was the case in previous periods, UTIs were the most frequent infections (63.4%), followed by CMV infection and disease (13.9%). The least frequent bacterial infections were: Clostridium difficile diarrhea (2 in the early period, 1 in the intermediate and 2 in the late period), septic arthritis (2 in the intermediate period), infectious endocarditis (1 in the early period and 1 in the intermediate period), osteomyelitis (2 in the intermediate period) and tuberculosis (1 in the early period and 1 in the intermediate). Fungal infections were the most infrequent: among them were disseminated candidiasis (1 in the early period) and UTI by Candida spp (1 in the late period).
A total of 137 opportunistic infections were identified (27.2% of total infections), 56.2% of which were CMV disease (77 episodes), followed by 33 cases of CMV infection (24.1%), 9 cases of Chagas’ disease (6.6%) and 8 cases of BKV infection (5.8%). In addition, among the opportunistic infections identified, 5 cases of aspergillosis were diagnosed (4 in the intermediate period and 1 in the late period), as were 2 cases of pneumocystosis (1 in the intermediate period and 1 in the late period) and 1 case of nocardiosis (in the late period).
Kidney recipients had pre-transplant serology positive for Epstein Barr virus (EBV) (92%), CMV (91.7%) and Chagas’ disease (6.9%). CMV reactivation was observed in 24.1% of the CMV seropositive patients, and Chagas’ disease reactivation was identified in 26.9% of the patients who tested positive for the disease before transplantation. No reactivation of other conditions was observed (Table 3).
Among patients with discordant serologies (seronegative recipients with seropositive donors), CMV transmission occurred in 56.5% (13/23) of the patients, and Chagas’ disease transmission occurred in 8.3% (2/24). Transmission of other donor infections to recipient was not observed (Table 4).
Nineteen patients died (5.1%) during the follow-up period. There were no significant differences in mortality among patients with at least one infection episode and patients who had none (6.4% vs 2.9%; p = 0.13). Likewise, no significant differences were found between the survival curves of both groups at 12-month follow-up (with infection 93.8% [95%CI 89.8-96.3] vs. without infection 97.1% [95%CI 92.3-98.9]; p = 0.14). When analyzing patients’ survival rate after the infection episode, differences in survival curves were found depending on the period in which the infection was diagnosed (early period 88.9% [95%CI 81.5-94.3] vs. intermediate period 96.1% [95%CI 89.9-98.5] vs. late period 100%; p = 0.048). Eleven deaths occurred in patients who had infections in the first period after transplantation (11.1%), 4 occurred among those who developed infections in the second period (3.7%), and no deaths occurred in the third period after transplantation.
Discussion
In our study, 62% of the patients had at least one episode of infection during the first year after transplantation, a rate which is in range with results published by other authors, where the incidence of infection ranged between 40-80%10-13. Among the patients included in our study, there were many with a history of previous renal transplant, and the average time on dialysis prior to transplantation was nearly 5 years. While these elements have been described as risk factors for developing infections, no significant differences were observed between both groups14. It was only observed that patients who developed infections were older and more frequently diabetic than those who did not. With regard to the transplant, as it has been described in other studies, we found that a history of deceased or expanded criteria donor graft and the delay in graft function was associated with an increased risk of infection15.
Moreover, we observed that prolonged post-transplant hospitalizations were most frequently associated with the development of infections. Such an increase in the length of post-transplant hospitalization might be due to the occurrence of medical complications, including infections, as a result of an increased length of exposure to nosocomial pathogens and the use of endovascular or urological invasive devices, as it has previously described10. When comparing the types of immunosuppressive maintenance and induction therapies, we found that the patients who used tacrolimus were at a higher risk of developing infections, as previously reported16. However, it must be considered that most patients in both groups did use tacrolimus, which is one of the most widely used immunosuppressive maintenance therapy along with mycophenolate.
In the bivariate analysis, patients who had a pigtail catheter inserted into the urinary tract appeared to be at a lower risk of infection. Actually, this is due to the fact that almost all transplanted patients who received an organ from a living donor (considered as a “protective factor” against infections) had a pigtail catheter, in comparison with a small percentage of patients who received an organ from a deceased donor (considered as a risk factor for infections). When adjusting these variables, the presence of a pigtail catheter was a clear risk factor for developing infections.
When surveying the epidemiology of infections according to the post-transplant period in which they developed, we noted that over half of them occurred between 30 days and 6 months after transplantation, as it has been described in other studies16,17. This is a period of maximum immunosuppression, and thus, it is reasonable to find it associated to a higher risk of developing opportunistic infections16. In our study, the most frequent cause of infection in this period was CMV and, to a lesser extent, Trypanosoma cruzi (agent of Chagas disease) and Pneumocystis. However, it should be noted that most bacterial infections during the first year after transplantation occurred in this period (pyelonephritis had the highest frequency).
Several studies indicated that bacterial infections are the most frequent in transplant patients, accounting for 50-70% of the episodes10,18. When these infections include bacteremia, mortality rate can be as high as 50% mortality. The main risk factors promoting their development are the use of urinary catheters, intravascular catheters, surgical procedures, history of CMV infection, and episodes of rejection19,20. As observed in our study, urinary infections have been reported as the main cause of bacterial infection in patients with renal transplantation21,22.
According to the literature, the lowest number of infection episodes during the first year post-transplant occur in the first 30 days after transplantation8,16. In our study, however, it was during the third period post-transplant when the lower number of infection events occurred, with a slight difference in frequency with respect to the first period. As previously reported, most infections occurring in the first month were of nosocomial origin, mainly secondary to donor-derived infections, surgical site infections, UTI and endovascular catheter-related infections10,17.
It has also been described that infections occurring after 6 months post-transplant tend to be similar to the ones developed by the rest of the population in general. This could be due to the fact that the period of highest immunosuppression, as well as the transplant-related risks factors, have been overcome8. In our study, the vast majority of infections occurring during this third period were community-acquired bacterial infections, mainly urinary infections and bacterial pneumonias. However, graft rejection episodes may increase the risk of opportunistic infections, mostly due to the need to increase the length of immunosuppression8,17. Opportunistic infections are of great relevance since their clinical manifestations tend to be atypical in transplant patients, and there may be delays in diagnosis and appropriate treatment23. In this study, about one-quarter of infections were secondary to opportunistic microorganisms, and they occurred between 30-180 days after transplantation. Many of them resulted from the reactivation of the recipient’s dormant viral infections, as well as from donor-transmitted infections. This demonstrates the importance of a complete pre-transplant screening of donor and recipient for the prevention and early detection of these kind of infections15,24.
Just like it is mentioned in the literature, CMV was the main opportunistic agent identified in this study25,26. CMV reactivation can develop as an infection (with a mere increase in the number of viral copies) or as a disease (with associated symptoms such as fever, general distress, leukopenia, thrombocytopenia or evidence of tissue invasion)26. As it has been noted by other authors, the prevalence of CMV positive serology was very high among kidney donors and recipients25. This a relevant aspect, since the greatest known risk factor for CMV disease is a mismatch between a CMV seropositive donor and a CMV seronegative recipient before transplantation27. In a study from Argentina, CMV accounted for 50% of the diarrhea episodes with identified microbiological agent in patients with kidney and simultaneous pancreas-kidney transplantation, being diarrhea one of the main manifestations of this infection28.
Chagas’ disease is an endemic zoonotic disease in several Latin American countries. It is estimated that in Argentina there are 2.5 million infected people29,30. There are some reports about the rate of reactivation and transmission during the post-transplantation period31-33. Riarte et al reported that, in a transplantation center in Argentina, 21% of renal transplant recipients with positive serology tests for Chagas experienced disease reactivation between 1989 and 199634. This percentage is very similar to the one observed in our study. On the other hand, those authors reported that donor transmission was close to 18%, in comparison with the 8% transmission rate observed in our study. This infection became of utmost importance since in the last decades it has spread to other continents via various means of transmission, such as vertical transmission, blood transfusion, and organ donation15,35,36.
Another important infection for renal transplant recipients is the one caused by BKV, which can be associated to a wide range of clinical manifestations, such as asymptomatic viruria, urethral stricture, interstitial nephritis, and graft nephropathy17,24,37. As in most studies, we found the frequency of BKV infection to be higher after the first post-transplant month, and mainly manifested as BKV nephropathy, albeit in a much lower percentage (1.4%) compared to reported rates (2-5%)16. In a study carried out in Argentina, with serial search in kidney transplant recipients, 12% of post-transplant patients were found to be infected with BKV38.
Tuberculosis is another infection that can develop in immunosuppressed patients. In Argentina, its incidence was 23.9 per 100 000 people in 2016, signaling an upward trend compared to previous years. In our study, there were two cases of tuberculosis, both of which occurred within 6 months post-transplant. While tuberculosis may be donor-transmitted or community-acquired, it usually develops as a reactivation of a latent infection in the recipient17,39. In one of the patients who developed tuberculosis, the result of the tuberculin skin test prior to transplantation was not recorded and in the other it was recorded as being negative. This test often gives false-negative results in anergic patients, such as those affected by chronic kidney diseases39. Thus, it is still possible that this transplant recipient had reactivated a latent tuberculosis infection. A large Argentine study conducted between the 1980s and 1990s reported 3.6% of tuberculosis in kidney transplant recipients with an average time of diagnosis at 13 months40.
Similarly to findings of other studies, infection episodes caused by fungi accounted for a small percentage, with Candida sp. as the main causative agent41. Another fungal agent isolated was Pneumocystis jiroveci, which accounts for severe pulmonary infections in transplant patients during the first 3-6 months after transplantation. However, the incidence of this infection has dropped substantially due to the use of antimicrobial prophylaxis with trimethoprim/sulfamethoxazole23. It should be emphasized that in our study we observed no case of toxoplasmosis, cryptococcosis, mucormycosis, histoplasmosis or EBV-related infections.
We conclude that infections in renal transplant recipients represent a very frequent and important problem in our setting. Thus, understanding the local epidemiology of infections and their potential risk factors is of special significance in order to design and implement appropriate measures for their prevention and/or timely treatment.