KEY POINTS
Current knowledge
• The COVID-19 pandemic has had a great impact in the field of lung function tests, due to the high capacity of SARS CoV2 infected patients to generate potentially infectious aerosols.
Contribution of the article to current knowledge
• New recommendations are incorporated to carry out PFTs in the context of a high and low phase of circulation of SARS CoV2 in the community for biosafety of both, pa tients and operators, as well as post-COVID-19 patients. Measures include ventilation of the environment, use of antimicrobial filters, protection of airways and mucous membranes, and hand washing.
The airborne spread is the main form of interhuman transmission of coronavirus, including the novel virus SARS CoV-2, which causes the disease known as CO VID-191-3. Contagion can occur through direct transmission of contaminated respiratory secretions from the patient to an uninfected subject, or, potentially, through unnoticed contact with these secretions deposited on inert objects4. The implicit risk of aerosolization of the SARS CoV-2 infec tive microdroplets while carrying out pulmonary function testing (PFT) has restricted significantly the activities in most Pulmonary Function Laboratories (PFL) since the start of the COVID-19 pandemic5,6. For these reasons, and because of the importance of PFTs for diagnosis, assessment, and follow-up of numerous respiratory and systemic diseases; it is necessary to agree on strategies that allow adapting PFL activities to the demands posed by the COVID-19 pandemic.
The purpose of this document is to update the recom mendations for the management of PFL in the context of the epidemic in Argentina, the initial version of which was timely published through the website of the Asociación Argentina de Medicina Respiratoria7, and the website COVID-19: Guidelines and Recommendations Directory of the European Respiratory Society8.
This document addresses essential aspects that should be considered for a rational basis of those recommenda tions, including the COVID-19 epidemiological evolution, the clinical condition of patients who require exploration of respiratory function during the epidemic, the PFTs recommended for their usefulness and safety, and the strategies to carry out them while minimizing the risk of the procedures. This document also includes the guidelines for the study of patients recovered from COVID-19.
Methodology
For these recommendations, similar documents were examined, including those issued by various respiratory medicine societies and institutions related to PFL activi ties5-15, government health and epidemiological agencies, and basic research studies on the dissemination of SARS CoV-2 and its conditioning factors. All the recommenda tions emanating from these documents were evaluated, in successive meetings, regarding their quality and rel evance. A committee was formed to draft the present recommendations, which were subsequently evaluated by an independent review committee.
These suggestions apply to any epidemiological phase, should it be primary or due to a new infectious outbreak. Reports of confirmed reinfection in the same patient are rare, so each case must be considered in particular16,17.
Conceptual bases for recommendations
1. Considerations about the epidemic evolution of COVID-19
To determine the feasibility of PFL activities, the local epidemiological evolution of COVID-19 must be taken into account, which might vary significantly, even between adjacent geographic regions and cities. For this reason, the situation in each different area must be carefully considered when deciding the level of PFL activity. In Argentina, this data is published on the website of the National Ministry of Health and also on regional health organizations, like the argentine website of the WHO/ PAHO14,18, where information can be obtained on the evo lution of disease prevalence and incidence rates, the curve of progression of confirmed cases by region, the basic reproductive number (R0) of the infection (which estimates the speed of spread of the disease in the population), and the expected time for cases duplication14,18,19. These and other variables should be analyzed jointly with the local infection control committee to define the epidemiological phase of the disease and agree on strategies to develop PFL activities.
For this work, the High Phase was defined as the local increasing or high stable epidemic situation (high plateau) of the disease, characterized by a high circulation of the virus in the community. The Low Phase was defined as the local decrease in the number of new confirmed cases or low stable situation (low plateau) when the probability of spread of the infection is lower.
During the High Phase, it is generally recommended not to perform PFTs. Only exceptional cases could be considered, in which functional examination is essential to take urgent therapeutic measures. While in the Low Phase, it is recommended to consider a gradual opening of the PFL, maintaining the concept of performing only those PFTs that are necessary for the diagnosis or to make medical decisions that cannot be postponed.
In both phases, it is advisable to defer all non-essential PFTs, postponing those requested for routine control of a known condition or that do not modify the clinical man agement. As it will be seen below, there must be written operating procedures including strategies to prevent a potential viral spread during the studies, as well as the use of personal protective equipment (PPE) elements and decontamination of equipment, according to local institu tional and health policies regarding the management of communicable infections.
2. Clinical conditions for conducting PFTs during the COVID-19 epidemic
It is not recommended to carry out of PFTs to any patient with a diagnosis of COVID-19, symptomatic or not, nor in those patients who meet clinical criteria for a suspicious case according to the definition of the National Ministry of Health in any of the epidemiological phases of the disease. There is evidence that 95% of patients who have suffered COVID-19 and have recovered (post-COVID) have a very low contagious potential beyond 20 days from the symp tomatic onset of the disease and for a period of at least 3 months21. In this context, it is reasonable to indicate a respiratory functional evaluation starting 30 days after the onset of COVID-19 symptoms based on a careful clinical evaluation and the absence of signs and symptoms of reinfection18,22, without further tests to exclude the pres ence of SARS-CoV-2. However, immunodeficient patients who have suffered from COVID-19 should be evaluated individually to exclude persistent infection23.
Presumedly, the evaluation of pulmonary function can be indicated in a post-COVID patient in at least two scenarios: 1) to determine the functional condition and its potential impact due to the sequelae of the disease in patients without previous respiratory disease and, 2) to evaluate an acute exacerbation or deterioration of a previously known concurrent disease (chronic obstructive pulmonary disease, pulmonary interstitial diseases, bron chiectasis, etc.). For the first scenario, evidence shows that an acute deterioration of the respiratory function can occur within the first 30 days from the onset of symptoms, and a slight improvement or stabilization is reached at around 3 months24. To know the remaining lung function, it is ideally suggested to assess it after 3 months of disease evolution25. In the second scenario, whenever possible, it is advisable to wait at least 30 days from the onset of symptoms24-26.
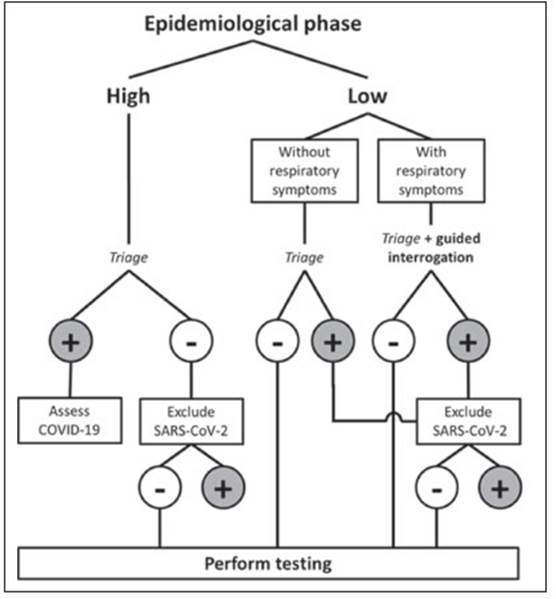
In patients who have not suffered from COVID-19 and require a PFT during the Low Phase, it is recommended to perform a triage questionnaire 24 to 48 hours before PFT execution to examine criteria for a suspected case of COVID-19. After an interrogation directed by trained PFL personnel, cases considered suspicious will not be able to access the functional study until the SARS-CoV-2 infection has been excluded. Directed questioning is in tended to discriminate between the usual symptoms of the patient’s underlying disease from those potentially linked to COVID-19 (Fig. 1).
The course of the epidemic varies temporarily and geo graphically, with periods of high and low local transmission rates that may differ between nearby regions. This reality must be recognized and evaluated with data provided by the local health authority to decide the conduct regarding carrying out PFTs. During the High Phase, in a patient with new or exacerbated respiratory symptoms, diagnosis of COVID-19 should be excluded using any of the validated and approved diagnostic tests such as nasopharyngeal swab and real-time polymerase chain reaction test (RT-PCR), saliva tests, etc. within 72 hours before the study6,11. During the Low Phase, following the healthcare center’s experience and the institutional infection committee rec ommendations, the absence of criteria for a suspected case of COVID-19 through a remote evaluation 24 to 48 hours before the study and the confirmation in person by trained personnel could be considered enough to perform a simple PFT with low contaminating potential. In contrast to the use of direct methods of exclusion of SARS CoV-2 infection, the success of this strategy will depend on the experience and the resources of the center as well as on the local prevalence of the infection5 (Fig. 1). It should be borne in mind that rapid tests, such as the nasopharyngeal swab and RT-PCR test, can have a false-negative rate of up to 30%27, depending on the moment in which they are performed and on some technical aspects of their obtention. Therefore, it is advisable to maintain certain protection and hygiene measures during the study as if it were a potentially infectious case.
Table 1 shows a list of situations proposed for the car rying out of PFTs. Each request must be evaluated by the professional in charge of the PFL, who will determine its feasibility based on biosafety regulations and will decide to accept or reject any or all of the requested tests. In the Low Phase, other indications for functional evaluation could be considered, such as clinical trials not related to COVID-19 or occupational health examination28.
3. Which PFTs can be performed during the COVID-19 pandemic?
The risk of contagion for the operator depends on factors such as ventilation and ambient air exchange, distance from the patient, proper use of PPE, the number of infec tious particles in the environment, and the possibility of their deposition in the airways and other mucous mem branes, infectious inoculum, among other factors29-31. Forced exhalation during PFTs generates the dissemina tion of respiratory secretions in the form of microparticles with a size of up to 20 μm in the environment close to the patient29. While the largest particles can be expelled up to 2 meters, the smallest (less than 5 μm) can remain temporarily suspended in the air, especially when the ventilation of the room is inadequate. During forced respiratory maneuvers, an expiratory flow greater than 700 L/min can be generated, with high dissemination of respiratory secretions in the form of microdroplets32-34. This phenomenon may be more accentuated with coughing and during the performance of PFTs characterized by a sustained increase in ventilation34.
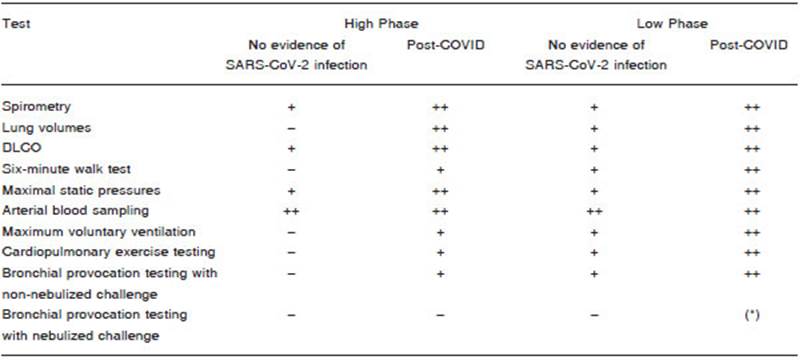
When considering the performance of PFTs during the COVID-19 epidemic, those studies that provide the most useful and specific information for the case being explored, and that at the same time are the simplest, shortest, and with the least respiratory secretions aerosolization should be indicated. Among them, spirometry, arterial blood gas, and measurement of carbon monoxide diffusing capacity (DLCO), which provide the most relevant information for clinical decision-making, can be carried out with the ap propriate precautionary measures for the epidemiological situation35. Practices such as bronchial provocation tests and cardiopulmonary exercise testing, which include repetitive forced breathing maneuvers or a sustained increase in ventilation, should not be performed during the High Phase of the pandemic. Some international recommendations5,6,9-11 maintain that these examinations should be postponed until the COVID-19 epidemic is in the phase of defervescence and epidemiological control. The measurement of total lung capacity (TLC) by any of its techniques can rarely be considered essential36, and it is advisable to be postponed until reaching the Low Phase (Table 2).
Regarding the 6-minute walk test, this working group recommends carrying it out preferably in external open spaces meeting the technical conditions required for this functional test (patios, open galleries, etc.)37,38. The use of corridors where patients and personnel circulate should be avoided to carry out the test; these could be considered to be performed in patients who meet post-COVID criteria. Depending on the risk assessment of contagion for the operator and third parties, the test could be carried out with or without a mask. When one of the previous options is chosen, the report should detail this modality, its influ ence on the Borg dyspnea scale, and the limitations of its interpretation against commonly used reference values.
In the Low Phase, the rest of the usual PFTs could be reintroduced, but always considering the absence of suspicion of viral infection, the use of appropriate PPE by the intervening health personnel, the hygiene measures of the equipment, and the execution of the studies in a place following the sanitary measures recommended below.
4. How to perform PFTs to reduce the risk of SARS-CoV-2 spread
4a. Conditioning of the waiting room and management of shifts: it is recommended that scheduling and pre-study indications should be done remotely (telephone, cell phone applications, web portals). It is also suggested to admin ister an evaluation questionnaire (triage) by telephone 24-48 hours before the study. A medical professional should repeat the questionnaire when the patient presents for testing. Having a form for registration and control of canceled and deferred appointments is advisable. At all times, social contact between staff and patients should be minimized. Spaced appointments must be granted, estimating a minimum time of 30 minutes from the end of the previous study to the beginning of the next one, to carry out the conditioning and hygiene of the study room.
Adequate ventilation and air renewal in the waiting room and study premises are important to reduce the permanence of infectious particles in the environment32,33. The waiting room must be ventilated through windows or forced ventilation of air to the outside. Air recirculation systems (fans, split-type air conditioning units) should not be used without adequate external ventilation, even at the cost of lower energy efficiency. Central air conditioning systems with an outside air intake are acceptable40.
The virus can remain viable on various inert materials4,41. Several reports have found viral particles on various surfaces exposed to aerosols generated by patients with COVID-1941, and this route of infection is experimentally possible. However, there are no documented cases to date of transmission by fomites42. The virus present on these surfaces can be eliminated with the use of the frequently recommended decontaminants41,43. To make the room easy and quick to clean, it is advisable to avoid the presence of non-essential items such as brochures, posters, cushions, covers, curtains, decorations, water dispensers, etc.4,44. Seats and other surfaces must be made of washable mate rial. Alcohol-based hand sanitizer or a washbasin with liquid soap and disposable paper towels should be accessible to waiting patients. Do not use bar soap or cloth towels. The number of people gathering simultaneously in the room must be kept to a minimum (a single companion per patient when necessary), with a distance of no less than 2 meters between patients (markings can be used on the seats) and ample circulation spaces. When this is not possible, the other patients will have to wait in another area of thecenter, or appointments will need to be granted at staggered hours. Patients and those accompanying them must wear a social mask at all times covering the nose, mouth, and chin45,46. It is advisable to place an information poster about hygiene measures in a visible place.
4b. Conditioning of the PFL or the room where PFTs are carried out: as well as the waiting room, it must be ventilated to the outside or have forced air ventilation to the outside. It is recommended that the windows are completely open, to guarantee the highest possible air exchange rate32,33. Recirculation systems, such as fans, should not be used without adequate ventilation to the outside. Centralized air conditioning systems with external air intake are acceptable, to which high-efficiency filters with the capacity to retain viruses and bacteria (HEPA) can be added and/or the installation of germicidal lamps of ultraviolet light type C40.
Locally installed air conditioning equipment (split type) could be used if it is possible to have external ventilation, as well as proper and regular maintenance and cleaning of filters. Consider maintaining temperatures between 24-27° C with relative humidity between 50-60%40,47. Ventilation towards internal corridors or waiting rooms is not acceptable. Poorly ventilated rooms pose a high risk of contagion, especially for staff; in these cases, health insti tutions should consider moving the PFL to other areas of the building that meet the ventilation conditions mentioned.
Although the ventilation of the test area is important, it is advisable not to carry out studies with flow sensors in the open air or areas with air currents, since air currents can generate artifacts in measurements due to instanta neous changes in BTPS (body temperature, pressure, water vapor saturated) correction.
It is convenient to minimize the number of exposed surfaces to facilitate cleaning and decontamination of the rooms where the PFTs are carried out. Sodium hypochlo rite, quaternary ammonium, or 70% alcohol solutions may be used. It is recommended that furniture is limited to the minimum, such as two chairs, a desk, and the equipment for measuring lung function, avoiding non-essential ele ments45,46. When entering the laboratory, the patient and his eventual companion should clean hands with alcohol-based hand sanitizer or soap and water.
There is evidence of airborne emission of particles from talking and breathing normally48. The permanent use of a mask can help minimize this aerosolization. The patient must be at all times with the facemask in place, removing it only to perform the maneuvers. In exceptional cases, the presence of a second operator may be considered.
Maneuvers necessary to carry out functional tests can spread a large number of infective viral particles into the environment32, thus the use of antimicrobial filters is es sential for containing them49-51. The antimicrobial filter must be specifically designed for pulmonary function tests, and be for single patient and study use51. The operator must control the correct labial occlusion over the interface with the filter to minimize the possibility of air leakage during the maneuver.
During the pandemic period, multiple devices have been developed to limit the risk of contagion for operators and patients. Among them, the use of sanitizing show ers, carpets with disinfectants, ozonizers, etc. can be mentioned. However, at present, we did not find evidence to support their usefulness, and the application of some of such devices has been discouraged52. Also, the use of bulkheads has been suggested as a physical barrier between the operator and the patient at the time of testing; although they have disseminated both in the commercial and health fields, no literature supports them and their implementation emanates from common sense. Its use would prevent the projection of large particles, but not of small particles that float in the environment and can reach the operator’s mucosae. Additionally, they can hinder interaction with certain populations that require assistance for mouthpiece placement or for achieving an adequate mouth closure, such as children, elderly, or neuromuscular patients. Therefore, their implementation is left to the criterion of the person in charge of the PFL and the institution. Their use does not prevent the need for ventilation, the use of antimicrobial filters, or the use of appropriate PPE for each situation, as we will see in the next section.
4c. Recommended protective equipment to carry out PFTs: the operator must use the PPE recommended for physical contact with a suspected COVID-19 patient fol lowing the regulations of the health authorities53,54.
PPE may include some or all of the following compo nents, depending on the risk of exposure:
- Scrub set for exclusive use in the PFL
- Closed footwear
- Long hair tied
- Disposable cap and boots
- Disposable water-repellent gown
- N95 or FFP2 face mask
- Three-layer surgical mask
- Closed goggles
- Facial shield
- Disposable gloves
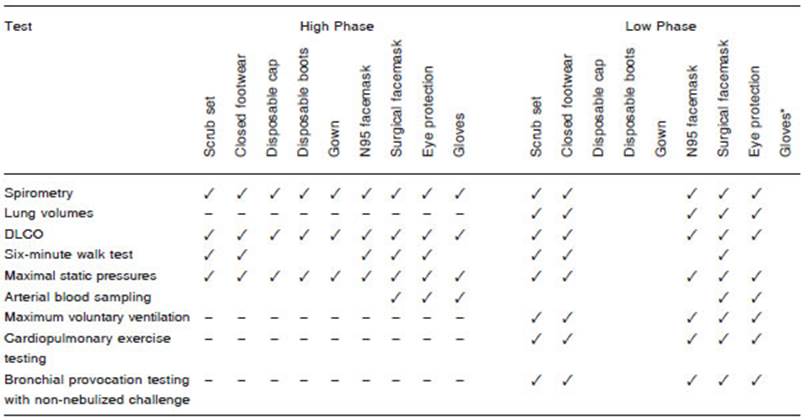
In high-risk scenarios (High Phase or with highly aero solizing tests), the use of PPE level 3 is recommended, which is intended for assistance and physical contact with symptomatic respiratory patients and exposure to potentially infectious respiratory secretions. This includes an N95 (or FFP2) and surgical mask over the former to prolong its durability, eye or face protection, gown, gloves, cap, and disposable boots. In the Low Phase, it could be considered to dispense some components of the PPE (Table 3). However, its implementation must be agreed upon with the institutional and/or local health authority.
The use of gloves is not essential unless the operator has skin lesions on his hands or wrists. Proper handwash ing between patients with soap and water or alcohol-based hand sanitizer is sufficient to eliminate the virus.
If more than one study needs to be carried out, more complete PPE should be used (Table 3).
During the administration of inhaled bronchodilators and in the waiting period between the pre- and post-bronchodilator phase, the operator must remain with the PPE on and inside the study room. The administration of inhaled bronchodilators carries a high potential for aerosolization of viral particles. The use of spacers or air chambers does not prevent the emission of microdroplets into the environment. If the patient comes into contact with the aerosol actuator (canister holder), it must be decontaminated after use. Spacers and air chambers must also be discarded or decontaminated following the regulations of the center55. The patient should stay in the study room between the phases of the study, and it is not recommended to intersperse patients between the phases of the bronchodilator test, as this implies decontamination and replacement of a new PPE for each phase.
Once the study is finished, the work area and any surface that may have come into contact with the opera tor or the patient should be cleaned. This cleaning can be carried out by the operator himself or by dedicated cleaning personnel, especially within a radius of two meters from the patient, taking into account the potential contamination with microdroplets of respiratory secre tions. It should be clarified that the cleaning personnel should enter the area with PPE identical to the one used by the operator. After cleaning the surfaces, the PPE components such as gloves, surgical mask, gown, cap, and boots should be discarded, and the goggles and face shield should be decontaminated with 70% alcohol or other suitable solutions56. A period of at least 30 min utes must elapse between patients to allow ventilation, cleaning, and the change of PPE. Surface cleaning can be done with sodium hypochlorite solutions. The correct dilution of sodium hypochlorite for disinfection of criti cal areas is 1000 ppm and is dependent on the initial chlorine concentration. For example, starting from an initial concentration of 55 g of chlorine per liter, make the following count: 1000 (ppm) X 1000 (ml of water in the sprayer) / 55000 (g of chlorine to mg) = 20 ml of chlorine in 1 liter of water. Chlorinates are used on surfaces that have already been cleaned and rinsed. They are only used in sprayers. The minimum concentration for virus elimination is 500 ppm57. This solution can be applied with a cloth and at least 5 minutes should be allowed to pass to achieve the elimination of the virus. For surfaces that could be damaged by sodium hypochlorite, it is recommended to use a concentration of 70% ethanol or quaternary ammonium solution, which are less corrosive.
Discussion
The COVID-19 pandemic has posed a significant chal lenge to biosafety during the performance of PFTs. Since airborne dissemination is the main form of interhuman transmission of this coronavirus, the implicit risk of the aerosolization of infecting droplets during the execution of PFTs has caused a significant limitation of activity in most of the PFL throughout the world. During the initial epidemic phase, the restriction of PFL activities was widely adopted as the best option (and perhaps the only one) to reduce the spread of the disease and the possibility of disease transmission to health personnel and patients5,6,58. However, the value of PFTs for the diagnosis, evaluation, and control of the evolution of respiratory and systemic conditions, has prompted the need to agree on strategies aimed at adapting the activities of PFLs to the require ments of the COVID-19 pandemic. However, to date, there is still no consensus on a solid and uniform way to maintain the biosafety of PFLs in this situation5-15. In fact, in some international guidelines and recommendations, decision-making is delegated to the experience and op erational availability of each PFL unit15. This shortcoming is due, at least partially, to the fact that even today the most important approach to PFL biosafety was focused on the cross-transmission of respiratory infections through the equipment59, relegating patient to operator transmis sion, as well as the indirect spread of potentially infectious secretions to other patients and health personnel from contaminated inert surfaces of the equipment and the place where PFTs are performed.
The objective of this document is to update the recom mendations for the management of PFLs in the context of the COVID-19 epidemic in Argentina based on an extensive review of other similar documents, published experimental and clinical studies, as well as on our initial recommendations issued in May 20207,8. New recom mendations have been incorporated in this edition for the management of PFTs in patients recovered from CO VID-19, the performance of PFTs in the context of a High and Low Phase of community viral circulation, as well as biosafety of the procedures involving patients, operators, and health personnel. However, the review of the informa tion consulted to support these recommendations shows the difficulty in obtaining a desirable level of category and quality degree. We think that this is partially due to the ethical limitation to carry out case-control studies to investigate the infectious potential of some PFL operating procedures, but also to the fact that in the last 20 years the PFL, in general, have relied on the biosafety of procedures in the very few reports of infections acquired through PFTs performance36,59. The reality of COVID-19 has abruptly ended with this fact, and, until we obtain more information about its impact in the field of PFL, the best available tools to conduct our task are the recommendations based on the quality of the available evidence, mostly indirect, as well as on the adherence to basic and rational measures for the prevention of transmission of respiratory infections.
Summary of recommendations for PFLs in the COVID-19 era
The following recommendations may vary after the issu ance of this document depending on new evidence and/or the local evolution of the epidemic, and eventual changes or modifications may be required in agreement with the institutional and/or local health authority, to adapt them to the reality of each workplace. For clarifications and annexes, refer to the full text.
a. To determine the feasibility of PFL activities, the lo cal epidemiological evolution of COVID-19 must be taken into account, which may differ significantly, even between adjacent geographic areas and cities. For this reason, the situation in each different area must be carefully consid ered when defining the level of activity of the PFL.
b. Prevalence and incidence rate of the disease, curve progression of confirmed cases by region, basic reproduc tive number (R0) of the infection (which estimates the speed of spread of the disease in the population), and time expected for duplication of cases should be analyzed together with the local infection control committee to define the epidemiological phases of the disease and agree on the strategies to develop the PFL activities.
c. The High Phase is defined as the local increasing or stable epidemic situation (high plateau) of the disease, characterized by a high circulation of the virus in the com munity. The Low Phase is defined as the local decrease in the number of new confirmed cases or low stable situ ation (low plateau) when the probability of spread of the infection is low.
d. During the High Phase, it is generally recommended not to perform PFTs. Only exceptional cases could be considered in which functional examination is indispens able to take urgent therapeutic measures.
e. During the Low Phase, it is suggested to consider the gradual opening of the PFL, maintaining the concept of performing only those PFTs necessary for the diagnosis or to decide on medical decisions that cannot be postponed.
f. It is recommended to defer all PFTs that are not es sential for diagnosis or when indicated for routine monitor ing of a known condition.
g. PFTs are not recommended in any patient with a current diagnosis of COVID-19, as well as in those who meet clinical criteria for a suspected case, in any of the epidemiological phases of the disease.
h. During the High Phase, in a patient with new or exacerbated respiratory symptoms, the diagnosis of COVID-19 should be excluded using any of the validated and approved diagnostic tests (nasopharyngeal swab and RT-PCR test, saliva, etc.), considering the possibility of performing them within 72 hours before the functional test.
i. In patients who have not suffered from COVID-19 and require a PFT during the Low Phase, it is recommended to perform a triage questionnaire 24 to 48 hours before the execution of the PFT to examine criteria for a suspected case of COVID-19. Suspected cases will not be able to access the functional study until the SARS CoV-2 infection has been excluded.
j. Patients who have suffered from COVID-19 pres ent very low infectious potential after 20 days of the symptomatic onset of the disease, for which its functional assessment is recommended starting 30 days from the onset of symptoms based on a careful clinical evaluation and the absence of signs and symptoms that could render suspicion of reinfection, without the need for further tests to exclude SARS CoV-2 infection.
k. During the Low Phase, following the healthcare cen ter’s experience and the institutional infection committee, the absence of criteria for a suspected case of COVID-19 through a remote evaluation 24 to 48 hours before the study followed by the confirmation in person by trained personnel could be considered enough to perform a simple PFT with low contaminating potential. In contrast to the use of direct methods of exclusion of SARS CoV-2 infection, the use of this strategy will depend on the experience and resources of the center, as well as the local prevalence of the infection.
l. When considering carrying out PFTs during the COV ID-19 epidemic, those studies that provide the most useful and specific information for the case being explored and that at the same time are simple and brief examinations with the least possible respiratory secretion aerosolization should be indicated.
m. Spirometry, arterial blood gas, and measurement of CO transfer with single breath (DLCO) are examina tions considered simple and can be performed with the appropriate precautionary measures for the epidemiologi cal situation. The possibility of carrying out the 6-minute walk test is considered when external open spaces are available that meet the technical conditions required for this functional test.
n. Some conditions that may require PFTs include pre operative risk assessment of lung resections, major sur gical procedures in patients with pre-existing respiratory diseases, evaluation of an exacerbation or progressive deterioration of known respiratory diseases, evaluation before a bone marrow or solid organ transplantation, and the implementation and control of therapy with potentially toxic drugs for the respiratory system.
o. Bronchial provocation testing and cardiopulmonary exercise testing, which include repetitive forced breathing maneuvers or a sustained increase in ventilation, should not be performed during the High Phase of the COVID-19 epidemic.
p. In post-COVID or Low Phase patients, the rest of the usual PFTs could be reintroduced, but always consider ing the absence of suspicion of viral infection, the use of appropriate PPE by the intervening health personnel, the hygiene measures of the equipment, and carrying out the studies in a place following the recommended sanitary measures.
q. Scheduling and pre-study indications to perform PFTs must be managed remotely (telephone, cell phone applications, web portals). It is recommended to administer an evaluation questionnaire (triage) by telephone 24-48 hours before the study.
r. Spaced appointments should be granted, estimating a minimum time of 30 minutes from the end of the previ ous study to the beginning of the next one to carry out the conditioning and hygiene of the study room.
s. The waiting room and the PFL or the place where the PFTs are carried out must have ventilation towards the exterior. Air recirculation systems should not be used. Likewise, there must be elements for hand washing or alcohol-based hand sanitizer and respect the general biosafety regulations at all times: social distance, chin strap or mask, staggered appointments, ample circula tion spaces.
t. Furniture limited to a minimum is recommended in the PFL, such as two chairs, a desk, and lung function measurement equipment, avoiding non-essential items. Upon entering the laboratory, patients should sanitize their hands with alcohol-based hand sanitizer.
u. The operator and the patient are the only ones who must remain in the PFL unless a companion is essential. The patient must be with the face mask in place, remov ing it only to perform the maneuvers required for PFT. In exceptional cases, the presence of a second operator may be considered necessary.
v. All PFTs must be performed with disposable antimi crobial filters. The antimicrobial filter must be specifically designed for pulmonary function testing, and be single patient and study use.
w. In the High Phase, the use of level 3 PPE is recom mended, which is intended for assistance and physical contact with symptomatic respiratory patients and expo sure to potentially infectious respiratory secretions. In the Low Phase, it could be considered to dispense with some components of the EPP in agreement with the institutional and/or local health authority.
x. During the administration of an inhaled broncho dilator, and in the waiting period between the pre and post-bronchodilator phase, the operator must remain with the PPE on.
y. After each patient, the study area should be cleaned within a radius of at least 2 meters using disinfectant solu tions. The operator can remove PPE only after cleaning is completed.