KEY POINTS
Evidence before this study
• The emergency management of the COVID-19 epidemic allows the administration of vaccines without having com pleted the phase 3 trials. Until February 2021, only ten countries have used the Sputnik V COVID-19 vaccine. Active and passive surveillance is essential for the noti fication of events supposedly attributable to vaccination and immunization.
Contribution
• This is the first study in evaluating the early safety of the Sputnik V COVID-19 vaccine under real conditions in Argentina. Among vaccinated healthcare workers, the early events supposedly attributable to the Sputnik V vaccine were fairly frequent but mostly mild.
The World Health Organization recommends vaccina tion against severe acute respiratory syndrome coronavi rus 2 (SARS-CoV-2) to mitigate the coronavirus disease 2019 (COVID-19) pandemic. Effective and safe vaccines will reduce COVID-19 related rates of disease, hospital ization, and death in the short term and help to gradually restore normal activities in our country1.
Sputnik V is a heterologous COVID-19 vaccine con sisting of two immunogenic components that are applied in two doses separated by at least 21 days. The first component contains a recombinant adenovirus type 26 (rAd26) vector and the second contains a recombinant adenovirus type 5 (rAd5) vector. Both vectors carry the gene for the full-length SARS-CoV-2 spike glycoprotein (rAd26-S and rAd5-S)2,3.
On December 23, 2020, the Argentine National Ad ministration of Drugs, Food and Medical Technology (ANMAT)4 performed a confidential technical report on the Sputnik V vaccine; the (Argentine) National Ministry of Health authorized its use under the label of an “emer gency authorization” within the framework of a national law specifically passed by the Argentine National Congress4. The Argentine National Ministry of Health5 developed a strategic plan to reach the quality standards of safety and efficacy for the entire Argentine territory, being vaccination free, voluntary, performed in stages, and independent of a history of having suffered the disease6.
For the surveillance of vaccine safety, the strategic plan prompts health effectors “to develop a specific plan for intensified passive and active surveillance of vaccine safety, which allows the continuous analysis of the noti fications of events supposedly attributable to vaccination and immunization (ESAVI)”6.
Faced with this epidemic emergency, which enables the administration of vaccines without having completed the phase 3 trials, it is essential to conduct safety research concurrent with the vaccination campaign. The Buenos Aires City´s Ministry of Health started, as a public policy, an active registry on surveillance of any vaccine applied to healthcare workers7. In this scenario of uncertainty, tools of information and communication technologies, like an ESAVI self-report form, are helpful to design collaborative epidemiological studies.
In Buenos Aires City, the vaccination campaign started on December 29, 2020, for healthcare workers from ei ther public or private effectors, with an initial endowment of 24 000 Sputnik V vaccine schemes, granted by the National Ministry of Health in two batches.
The present study is a preliminary analysis of an ongo ing multicenter study conducted in private health institu tions in Buenos Aires City. The study is aimed to describe the incidence of ESAVI in healthcare workers after immu nization with the first component of the Sputnik V vaccine. A previous version of this manuscript has been shared in medRxiv, a free online archive and distribution server for complete but unpublished manuscripts (preprints) in the medical, clinical, and related health sciences8.
Materials and methods
A prospective cohort represented by healthcare workers im munized with the Sputnik V COVID-19 vaccine was carried out blinded. The surveillance period was 72 hours post-immunization. A self-report form was sent by email up to three times. Those workers who did not respond to the email were contacted by a nurse. Those reactions that were considered ESAVIs after medical evaluation were reported to the Argen tine integrated health information system (SISA).
Quantitative data were expressed as mean and standard deviation or median and interquartile range (IQR) according to distribution. Qualitative data were expressed as absolute or relative frequencies.
The age categories were defined in 10-year groups, except for individuals between 18 and 30 years old who were grouped in one category. To allow external comparisons with other publications, we also defined a dichotomous classification in individuals ≤ 55 or > 55 years old.
The incidence rate of ESAVIs and 95% confidence intervals (95% CI) were estimated. The Kaplan-Meier estimator and plotter were performed by gender and age groups. Factors associated with the occurrence of ESAVI were evaluated using the Cox regression. The crude and adjusted Hazard Ratio (HR) were expressed with 95% CIs. A p level < 0.05 was considered statistically significant. The analysis was carried out with the R software version 4.0.3.
The protocol was approved by the Research Protocol Eth ics Committee of the institution where the study was carried out under number 3876 and was registered in ClinicalTrials.gov Identifier: NCT04738435.
Results
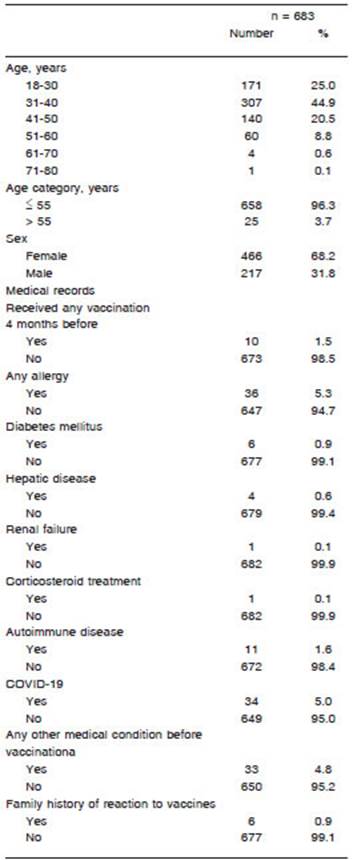
A total of 707 healthcare workers received the first com ponent of the Sputnik V COVID-19 vaccine in Buenos Aires, Argentina, between January 5 and January 20, 2021. Six hundred and eighty-three answered the self-report form, representing a 96.6% response rate. The median age was 35.0 years (IQR 30.5-42.0) and 67% were female. Thirty-four out of the 683 healthworkers had previously had COVID-19 confirmed by real-time polymerase chain reaction. The test had been performed in case of symptoms or close contacts. Of these 34, the median time from COVID-19 positive test to vaccination date was 5.3 months (IQR 4.2-6.9), and none of them had active COVID-19 infection at the time of receiving the first vaccine component. In this institution, antibodies against SARS-CoV-2 are not routinely performed in healthcare workers. Table 1 shows the characteristic of vaccinated healthcare workers.
Four hundred and eighty-seven out of the 683 health care workers (71.3%) reported at least one ESAVI. The incidence rate was 6.3 per 1000 person/hour (95% CI 5.8-6.9). Regarding the clinical evolution of the ESAVI, 422 (86.6%) vaccinees had restitutio ad integrum, 25 (5.1%) needed medical assessment, and one was hospitalized due to an acute abdomen that resolved favorably without surgery. Three had COVID-19 diagnosed within 72 hours of vaccination. Local and systemic reactions are shown in Figure 1.
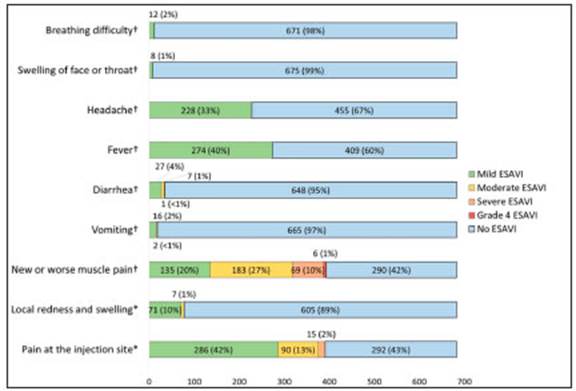
Fig. 1 Local and systemic reactions in healthcare workers after the administration of the first com ponent of Sputnik V COVID-19 vaccine.
The cumulative incidence of ESAVI at 72 hours was 61.7% (95% CI 58.1-65.4); it was higher in women (66.4%; 95% CI 62.1-70.7) than in men (51.4%; 95% CI 44.9- 58.2). Figure 2 shows ESAVI cumulative incidence in males and females.
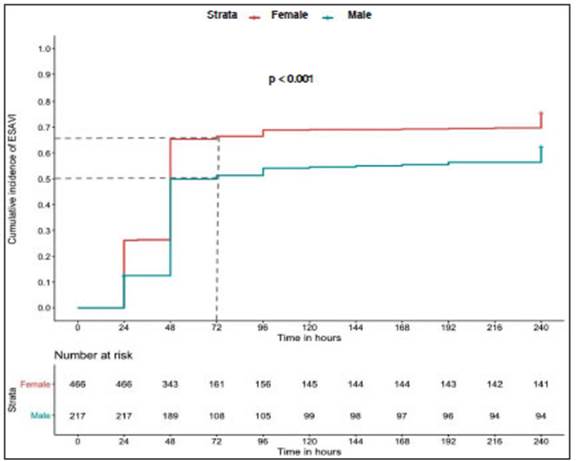
Fig. 2 Cumulative incidence of ESAVI in males and females after the administration of the first component of Sputnik V COVID-19 vaccine.
Regarding age groups, the incidence rate of ESAVI was higher in vaccinees aged up to 55 years than in those older than 55 years old, (72.8% n: 479 versus 32% n: 8). The cumulative incidence of ESAVI at 72 hours was 28.0% (95% CI 18.9-60.4) in people older than 55 years and 63.0% (95% CI 59.3-66.7) in those who were 55 years old or younger. Figure 3 shows ESAVI cumulative incidence in these age groups.
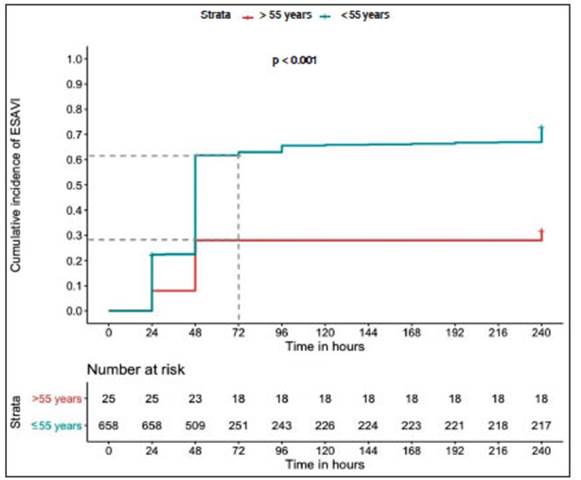
Fig. 3 Cumulative incidence of ESAVI in healthcare workers by age group after the administration of the first component of Sputnik V COVID-19 vaccine.
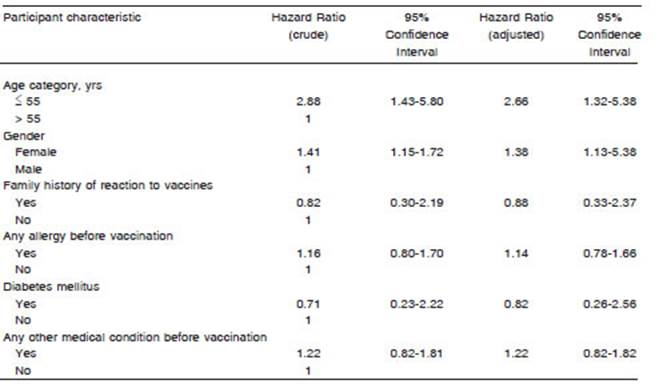
Both age and sex were factors associated with the incidence of ESAVI regardless of health condition before vaccination. Table 2 shows Cox regression analysis.
Discussion
In our study, the incidence of ESAVI with the Sputnik V vaccine among the 683 vaccinated healthcare workers (71.3%) was 17 times higher than the 4.7% disclosed in the “5th Surveillance Report on the Safety of Vaccines of the National Ministry of Health”9 and similar to that reported in the interim analysis of the Sputnik V COVID-19 vaccine phase 3 trial (64.7%)3. Only one person (0.1%) required hospitalization in our study compared to 45 (0.3%) in the Sputnik V phase 3 study3.
The incidence rates of pain at the injection site, fever, and headache in our study were within the range of values reported in previous Sputnik V vaccine studies2. However, we found muscular pain reported more than twice the rate reported in phase 1 and 2 of the Sputnik study (58% vs. 23% respectively); no data on muscular pain was reported in the interim analysis of phase 3 of this vaccine trial3.
In our study, ESAVIs were more frequent in females. Similar to the study on the BNT162b2 mRNA COVID-19 vaccine (Pfizer)10, we observed a lower incidence of ESAVIs in older people; adverse events among older than 55 years were 71% for Pfizer vaccine compared to 32% for Sputnik V in our study whereas in 55 years old and younger adverse events were 83% vs. 72.8%, respectively9,10. In phase 3 interim analysis of the Sputnik V vaccine, the authors found higher levels of antibodies specific to the receptor-binding domain of SARS-CoV-2 glycoprotein S among the younger participants, compared to the older ones; however, they did not compare ESAVIs between age categories to relate the reactogenicity with the adaptive immune response to the vaccine3. We also found a higher incidence of ESAVIs among women com pared to men (65% vs. 50%) but did not find a comparison of ESAVIs by gender in other studies.
To understand the frequency and kind of symptoms attributable to vaccination, it is important to analyze the reactogenicity11,12. Reactogenicity refers to a subset of symptoms or reactions occurring shortly after vac cination, which are regarded as physical signs of the inflammatory response to the vaccine. These reactions usually are mild and self-limited and rarely have serious consequences. Reactogenicity may contribute to misper ceptions (prejudices) against vaccination. A person could perceive a vaccine as excessively reactogenic and could reject additional doses, or a healthcare professional could advise against it. Reaching and maintaining a high vaccine coverage is critical to the success of vaccination programs and this kind of misperceptions jeopardize the endeavour13.
On the other hand, a relation of reactogenicity with early innate and adaptive responses has been proposed but a predictive association between them has not been demonstrated; thus, no evidence has as yet been ob tained of the known concept “no local pain, no gain in immunity”14.
Recently, Sadoff et al published preliminary results of phases 1 and 2 of the Ad26.COV2.S vaccine (Janssen/ Johnson & Johnson)15. This randomized clinical trial en rolled 805 healthy volunteers in two age cohorts to assess vaccine safety, reactogenicity and immunogenicity14. As we found in our investigation, they showed that the rate of adverse events decreased with the increase of age. Local and systemic reactions were generally resolved within 24 hours. Systemic reactions were largely responsive to antipyretic drugs. These authors found that the incidence of systemic adverse events among participants aged be tween 18 and 55 years was much lower after the second shot than after the first, regardless of having used the low or high dose, a finding that contrasts with observations made for messenger RNA-based vaccines, for which the second shot has been associated with increased reactogenicity15.
It is worth mentioning that in the publication of the University of Oxford with the AstraZeneca vaccine, a subgroup inadvertently received half a dose in the first shot and a full dose in the second. During the follow-up, this subgroup had not only less reactogenicity but also higher immunogenicity16,17.
In our study, the response rate to the self-report form was very high, in part because an epidemiologist explained the ESAVI collection tool to the participants at the time of vaccination and a nurse reinforced the explanation by tele phone. On the other hand, items questioned by the media, such as the origin of the vaccine or the delayed publication of the phase 3 trial results, could have prompted a greater report rate. The self-report form used in this study is being revised for validity and reliability.
Further research is needed to better understand the immune mechanisms involved in reactogenicity and epide miological surveillance of long-term adverse events. Even though there is evidence that the immune response is lower in older adults and males18, 19, understanding whether the efficacy of the vaccine is lower in people with low reacto genicity will be useful to define the vaccination scheme.