KEY POINTS
Current knowledge
• Individuals with hematological diseases have a variable immunocompromise because of the disease itself and the treatment they need. These patients have a higher morbidity and mortality by SARS-CoV2 than general population.
Contribution of the article to the knowledge
• In a population of 491 individuals with hematological diseases and SARS-CoV2 infection, the mortality at day 30 was 20.8% and raised to 63.2% in patients requiring mechanical ventilation.
• Factors associated with inferior overall survival were: comorbidities, status of hematological disease at coro navirus infection and a history of chemotherapy.
In December 2019, an outbreak of a respiratory illness caused by a new coronavirus was detected in China. This virus was called Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV2). This new type of respiratory disease (COVID-19) is characterized by the high human-human transmission1. On January, 30th 2020, the Chief Executive Officer (CEO) of the World Health Organization (WHO) declared that the outbreak of the new coronavirus represented a public health emergency and on March, 11th 2020, it was declared a pandemic by the WHO2. To date, 88 387 352 worldwide cases have been confirmed and there have been 1 919 204 deaths caused by CO VID-193. In Argentina, there were 1 703 352 number of cases and 41 273 deaths from COVID-19 until December, 16th 2020. The first identified case in the country was in March, 3rd 20203,4.
The Italian National Institute reported a case fatality rate of 7.2%, based on 22 512 cases. These deaths were mainly observed in old, male patients with multiple comorbidities; of this series, the 20% of the deceased were cancer patients5. A meta-analysis of 22 studies reported a mortality of 21.1% in cancer patients6. On the other hand, adult patients with hematological malignancies have a higher mortality due to COVID-19 than the general popula tion7. These numbers have been variable: between 14% and 36% depending on the characteristics of the disease and the geographic location of the published series8-14.
Since the beginning of the pandemic, recommenda tions have been issued by different scientific societies. The aim is to reduce the probability of infection and death by COVID-19 in patients with hematological and oncologi cal pathologies, based on the immunosuppression that these individuals have and which is due to their underly ing pathology and the treatments they receive15-21. The negative impact of the treatments could depend on the corresponding diagnosis, but patients with hematological malignancies constitute, in turn, a group with potential worsening of the disease due to the delay or the lack of a timely treatment. Thus, in pandemic times a careful bal ance of the risk and benefit of a specific treatment must be considered22,23.
In Argentina, in 2009, during the Influenza A (H1N1) epidemic, the incidence of pneumonia among patients with neoplasms was 66%, and the mortality was 18%24. Therefore, it is a priority to know the clinical character istics and the evolution of patients with hematological pathologies in Argentina to establish guidelines for the care and treatment of these individuals. The Argentine Society of Hematology has designed a survey to know the geographical distribution and the clinical characteristics of SARS-CoV2 infection in patients with hematological dis eases in our country. The primary objective of the present study was to evaluate the survival at day 30 of patients with hematological diseases and who tested positive for SARS- CoV2; and the secondary objectives were: (i) to describe the clinical symptoms, the underlying diagnosis, the comorbidities and the treatment of the underlying pathology of patients with SARS-CoV2 infection; (ii) to determine the factors linked with the deaths caused by COVID-19; (iii) to determine the need of hospitalization in an intensive care unit (ICU) and invasive mechanical ventilation (IMV) in this patient population.
Materials and methods
A survey was designed through an electronic form by the first author. The survey input began on 04/19/2020 and the data analysis was made on 12/7/2020. This survey was ret rospective since it had to be completed at least 30 days after diagnosing SARS-CoV2 infection. All national specialists in Hematology were invited to participate through the Argentine Society of Hematology.
The eligibility criteria were: patients of all ages with a diagnosis of a benign or malignant hematological pathology and SARS-CoV2 infection confirmed by PCR technique. Those cases that had a data absence about the patient evolution were excluded. Among the variables studied, death from COVID-19 - according to WHO - was included as a result variable: death resulting from a disease clinically suitable with a probable or confirmed case, unless there was a clear alternative cause of death that could not be related to the disease due to COVID-19, and there should not be a complete recovery period between the disease and the death. It was also included as an exposure variable: confirmed COVID-19 infection. In relation to the demographic variables and patient data, the following items were included: patient initials, age at the time of diagnosis of SARS-CoV2 infection, gender, province of origin, diagnosis of hematological pathology, date of diagnosis of hematologic pathology, disease status at the time of SARS-CoV2 infection (remission, active disease), co morbidities (smoking, asthma, diabetes, high blood pressure, obesity, chronic obstructive pulmonary disease, solid tumor, heart failure, ischemic heart disease, arrhythmia, chronic kidney failure, stroke, liver cirrhosis, autoimmune diseases, history of chemotherapy, date of the last cycle and scheme received, history of hematopoietic stem cell transplantation (SCT) (autologous/allogeneic) and date of SCT. Regarding the SARS-CoV2 infection, the variables were: symptoms, date of onset of symptoms, presence of fever, cough, odynophagia, dyspnea, diarrhea, evidence of other symptoms, pneumonia, computed tomography, bronchoalveolar lavage, coagulopathy, hospitalization requirement, date of admission to hospitaliza tion, discharge date, need for ICU, mechanical respiratory as sistance requirement, treatment received (hydroxychloroquine/ azithromycin-lopinavir/ritonavir-steroids- others), death and date of death or last update.
The continuous variables are presented as median and interquartile range (IQR) and were compared using the Mann Whitney test. The categorical variables are presented as rela tive frequencies and percentages; also, the chi-square test or Fisher’s exact were used for comparisons. The overall survival (OS) was analyzed using Kaplan-Meier curves and compared using the log-rank test. For the univariate and multivariate comparisons, the respective unadjusted and adjusted HR were obtained through COX regression, with their respective 95% confidence intervals (95% CI). Factors with a p value of less than 0.05 in the univariate were entered in the multivariate analysis performing Cox regressions to obtain the final model. The statistical significance was defined as a p value <0.05 and the statistical analysis was carried out using the Easy R statistical program Easy R25.
The study was carried out in full accordance with the current national and international regulations: Declaration of Helsinki of the World Medical Association in its most updated version, resolution 1480/2011 of the National Ministry of Health and the Law 25326 on Protection of Personal Data. All the study data was treated with the utmost confidentiality and anonymously, with restricted access only for the authorized personnel for the purposes of the study and, once more, in accordance with the regulations above mentioned. The study was approved by the Comité Institucional de Ética de Inves tigación en Salud (Health Research Ethics Committee) of the Hospital Privado Universitario de Córdoba.
Results
There were 428 response forms received, and 9 of them were duplicated, so they were excluded. This leaves 419 cases suitable for analysis.
The cases were registered between 04/19/2020 and 12/07/2020. The vast majority of them belonged to Buenos Aires Province (n = 135) and to the Autonomous City of Buenos Aires (n = 128), this was followed by the prov inces of Córdoba (n = 38) and Mendoza (n = 34) (Fig. 1). The demographic characteristics and comorbidities are expressed in Table 1.
In reference to the clinical data and the disease due to COVID-19, it was observed that the majority of the 419 patients studied had a diagnosis of hematological malignancies (n = 383; 91.4%). The most frequent diag noses were non-Hodgkin lymphoma, acute leukemia, and multiple myeloma/monoclonal gammopathies (Table 1). The average age was 58 years old; only seven (1.7%) cases younger than 18 years old were registered, 306 were older than 40 years old (73%) and 92 (21.9%) were older than 70 years.
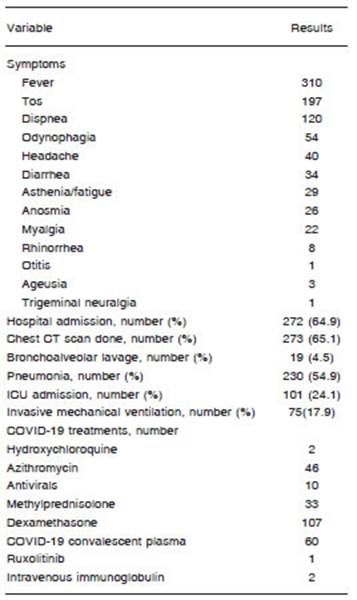
From the 419 assessable patients, 358 had symptoms (85.4%). The most frequent symptom was fever. In addi tion, the following syndromes associated with COVID-19 were described: idiopathic thrombocytopenic purpura (n = 1), hemolytic anemia (n = 3), coagulopathy (n = 4) and deep vein thrombosis (n = 10). More than half of the patients required hospitalization (n=272, 64.9%) and 101 were hospitalized in ICU (24.1%) (Table 2). A variety of treatments were reported and the most common one was dexamethasone (Table 2).
Of the total number, 87 patients died in an average of 16 days (range 0-100). The overall survival (OS) at day 30 was 80.2% (95% CI 75.6-84) (Fig. 2). Patients hospital ized for COVID-19 had an OS at day 30 of 74.2% (95% CI 67.9-79.5). Those who were admitted to the ICU had an OS at day 30 of 46.6% (95% CI 36.2-56.3). Those 75 cases who required IMV, the 30-day OS was 36.8% (95% CI 25.8-47.8). The 30-day OS of patients with malignan cies and benign diseases was 80.6% (95% CI 75.9-84.6) and 84.8% (95% CI 67.2-93.4) (p = 0.257), respectively. The mortality data due to disease is shown in Table 3; it excludes those with an allogeneic stem cell transplanta tion (SCT). The factors linked with the OS in the univariate analysis were: age, the presence of comorbidities, the disease state at the time of SARS-CoV-2 infection and the history of chemotherapy.

Table 3 Survival according to the diagnosis (patients with a history of allogeneic transplant were excluded)
Except for age, the remaining variables retained their statistical significance in the multivariate model (p < 0.05): the presence of two or more comorbidities (HR 2.271), an antecedent of previous chemotherapy (HR 2.548) and the presence of active hematological disease (HR 3.104) (Table 4). Afterwards, an index was constructed to predict survival at day 30 considering these significant parameters, awarding one point to the variables two or more comorbidities and an antecedent of chemotherapy, and two points to the presence of active disease, accord ing to the adjusted HR. Mortality was similar between those groups with one and two points, so four groups were individualized with a significant difference in the overall survival at day 30 (p < 0.001): 0 points (n = 21; 100%); 1-2 points (n = 201; 87.5%; 95% CI 81.4 - 91.7); 3 points (n = 152; 76.1%; 95% CI 67.3 - 82.8); and 4 points (n = 45; 49.6%; 95% CI 32.8 - 64.2); (Fig. 3).
There were 19 registered patients who had COVID-19 infection after a SCT (average age 49 years old; IQR 36-53): 10 with identical related donors, six with partially identical related donors and 3 with unrelated donors. In five of the 19 cases, the SCT was performed during the 2020. Of those 19 patients, 15 presented symptoms and 14 required hospitalization: six in ICU and five required IMV. The 30-day OS was 70.3% (95% CI 30.1-80.7). Regarding the six deaths, two of them presented a septic shock, one also presented other transplant complications - such as veno- occlusive disease and graft failure - one presented a relapse of the disease and another patient had severe COVID-19 in the context of chronic graft ver sus host disease (GVHD). All the deceased patients were receiving immunosuppression at the time of COVID-19 disease (Table 5).
Discussion
We present the data of 419 patients with a hematologi cal pathology and who had COVID-19 in Argentina. The average age of the individuals was 58 years old, the aver age number of comorbidities was one, the majority had a diagnosis of malignancy, the majority were under an active treatment at the time of the infection and there were 14.6% (n = 61) asymptomatic patients reported. The mortality at day 30 was 20.8%. The most important adverse factors linked with survival after COVID-19 were: comorbidities, chemotherapy treatment, and active disease at the time of the infection.
Several groups presented the data and the evolution of COVID-19 in the hematological population. The ASH Research Collaborative COVID-19 Registry for Hema tology reported data from 250 cases with a malignant hematological pathology, 78% of them were older than 40 years and 36% were older than 70 years. In those series, only the 3% were asymptomatic, 54% was hyper tensive, 33% were diabetic, and 25% were smokers or ex-smokers. Moreover, the most frequent malignancy was acute leukemia. The mortality of patients with moderate to severe COVID-19 who required hospitalization was 28% and increasing to 42%9,10. Our series differ from ASH’s in terms of the proportion of asymptomatic cases, the supe rior proportion of comorbidities and the inferior mortality, probably because we included patients with all types of hematological pathologies and a younger population. The series published by the Italian Hematology Alliance on COVID-19 registered a number of 536 patients with COVID-19 and who also had hematological neoplasms (average age 68 years old; average comorbidities: four); the mortality of the whole group was 37%7. Despite be ing older and comorbid individuals, the mortality of those admitted to the ICU was 63%7, similar to the one in our series. The Asociación Madrileña de Hematología y He moterapia [The Madrid Association of Hematology and Hemotherapy] analysed data of 697 patients with hema tological malignancies (average age 72 years). In 62% of the cases, COVID-19 disease was severe / critical in 62% and mortality was 33%8. Like in our series, the presence of more than two comorbidities was associated with a higher mortality. Other significant factors were the diagnosis of acute myeloid leukemia (AML) and active treatment with monoclonal antibodies, while chronic myeloproliferative neoplasms and the treatment with hypomethylating agents were associated with a lower mortality8.
Considering the mortality due to disease, we found that acute leukemias and plasma cell neoplasms were associ ated with higher mortality. This observation was made by other authors8. Of the group of patients with plasma cell neoplasm, 16 out of 70 died due to COVID-19 (22.8%). In the series by Chari et al. and according to the International Myeloma Society, of 650 individuals with multiple myeloma (average 69 years old), 33% died after the infection with SARS-CoV2, although the geographic variability was very high and the mortality ranged from 27% to 57%. The factors associated with higher probability of death were: age, high-risk myeloma, renal involvement and suboptimal myeloma control; the history of autologous SCT had no impact22. In the UK series, the mortality reached 54.6% in patients with symptomatic myeloma and who were under systemic treatment at the time of SARS-CoV2 infection26.
In individuals with Philadelphia negative myeloprolif erative neoplasms, the mortality in our series was 15.4%, which is lower than the 28.6% reported in the series by Barbui et al. (n = 175), probably due to the higher num ber of cases in this series27. Several authors found that patients in the chronic phase of chronic myeloid leukemia (CML) and who were receiving treatment with tyrosine kinase inhibitors were not at an increased risk of getting COVID-19 infection or death28-30. In the group of Argentine patients with CML, five out of 24 cases died. However, three of them were in the blastic phase and two in the chronic phase had four and three comorbidities, respec tively. Our series also included 36 individuals with a benign hematological pathology; five deceased cases were in this group. Few series included patients with benign he matological diseases. Fox et al. studied 55 hematological patients with COVID-19. In this series, three cases had a benign pathology and one of them died from COVID-199,10.
Of the 419 patients included in our study, 19 had a his tory of allogeneic SCT and, in this group, the mortality was higher than in the whole cohort since it was 30% at day 30. All the cases that died were transplanted in the years 2019 and 2020 and were under immunosuppressive agents at the moment of the infection. The data about the impact of COVID-19 in the context of SCT is variable. Researchers at the Memorial Sloan Kettering Cancer Center studied 77 individuals who received cell therapy (allogeneic, au tologous, and CAR-T transplantation) and who also had SARS-CoV2 infection. The overall mortality of hospital ized patients with an active malignancy was 41% and of hospitalized patients without an active malignancy was 21%. The comorbidities were another determining factor of evolution. Antibodies against SARS-CoV2 after infec tion were developed in 66% of the cases31. Sultan et al., described seven cases with a history of allogeneic SCT and who had SARS-CoV2. All of these were in complete remission and receiving immunosuppression, and 3 of them had GVHD. There was no mortality from COVID-19 in that series32. The Spanish group also found a lower mortality in SCT recipients than in non-SCT33. In contrast, a mortality of 43% was reported by Kanellopoulus et al. in another series of seven allogeneic SCT recipients34. The 30-day overall survival of 70% in our cohort is similar to a recent CIBMTR report with 318 transplanted patients with COVID-19, and to the Italian and North American cohorts7,31,35,36. In the CIBMTR report, at 30 days after the diagnosis of COVID-19, the overall survival was 68% for recipients of allogeneic SCT and 67% for recipients of autologous SCT. On the one hand, among the allogeneic SCT recipients, the factors associated with a poor outcome were: 50 years old or older, male sex and the develop ment of COVID-19 within 12 months of transplantation36. On the other hand, among the autologous SCT recipients, a disease indication of lymphoma was associated with a higher risk of mortality compared with plasma cell disorder or myeloma36.
The impact of COVID-19 in the present study was as sessed in terms of hospitalization and mortality. However, other factors that may impact survival of this population were not assessed. In the study by Albiges et al., 178 cancer patients managed at the Gustave Roussy Cancer Center were studied; of them, 17.8% were hematologi cal patients. Mortality was 17.4%, the average delay in cancer treatment was 20 days in 41% of the cases, and the therapeutic strategy had to be adapted to the clinical situation of the patient in 30% of the cases37.
Within the treatments used against COVID-19, in our cohort, most of the cases received steroids. Only the 11% received azithromycin and 0.5% hydroxychloroquine compared with ASH’s registry that showed 50% y 64% re spectively9. It is important to know that to date, no standard treatment has been established for immunosuppressed individuals with COVID-19.
One of the main limitations of our study resides in the fact that it is a collaborative registry, so there might be an interobserver variability and, as the treatments are not standardized, there may be an impact on the patient’s clinical results which may constitute a bias. However, we consider that the data obtained provides knowledge about the impact of an emerging pathology in our population, thus making it possible to evaluate those results that can modify screening COVID-19 and treatment decisions. Our survey allows us to demonstrate the greater severity of SARS-CoV2 infection in hematological patients and to identify the clinical variables associated with a worse evo lution. As a consequence, in order to reduce the morbidity and mortality of patients with hematological diseases, some prevention methods should be considered; the most important one is to reduce the risk inside the hospital and the exposure outside it and to insist on the education about the use of masks, hand washing and distancing.