KEY POINTS
Current knowledge
• The coronavirus-2 of the severe acute respiratory syn drome (SARS-CoV-2) has been overwhelming the sani tary systems worldwide through a new form of respiratory illness called coronavirus disease 2019 (COVID-19).
• Few data are known regarding the repercussion in the pulmonary function of patients who had severe or critical COVID-19 pneumonia.
Contribution of the article to the current knowledge:
• The presence of abnormal gas exchange is the main characteristic of patients with COVID-19 pulmonary sequelae.
• More than half of the patients referred dyspnoea and had DLCO and KCO values lower than those who did not have this symptom, making gas exchange alterations the origin of dyspnoea.
• The evolution towards pulmonary sequelae was not as sociated with the severity of the disease.
In December 2019 a new pneumonia outbreak due to a newly identified coronavirus was informed in the city of Wuhan, Hubei, in China. The etiological agent was named coronavirus 2 of the severe acute respiratory syndrome (SARS-CoV-2) and has been overwhelming the sanitary systems worldwide through a new form of respiratory disease called COVID-191,2. Although the majority of patients have an asymptomatic or mild illness, the total number of severe cases and the mortality rates are un fortunately high3-9. In our hospital until the end of October 2020 11, 614 cases of COVID-19 were diagnosed with 1,252 hospitalizations (10.8%) and 254 deaths (2.2%). The recovery time of the acute disease seems to be around two weeks in mild cases and three to six weeks in severe illness, not being enough information regarding the possible pulmonary sequelae. There are few publications assessing the repercussion in the respiratory function in those patients who suffered a significant disease and there is practically no data about those who had critical forms of the illness10-14.
The objective of our study was to describe the pulmo nary function in patients who had severe pneumonia due to COVID-19, including the critically ill, after discharge and within 8 to 12 weeks after diagnosis. Establish if there exists an association between the pulmonary function and the severity of the disease, the presence of comorbidi ties, the tomographic involvement and the persistence of dyspnoea.
Materials and methods
Analytic, observational, and cross-sectional study. Fifty-five consecutive patients with severe and critical pneumonia due to COVID-19 disease were included, confirmed by the real-time reverse transcription-polymerase chain reaction test (rRT-PCR) nasopharyngeal swab specimens, sent for evaluation of the pulmonary function from September 7 to December 31, 2020. According to the provisional guidelines of the World Health Organization (WHO) severe pneumonia was considered when one of the following criteria was included: breathing rate > 30 breaths per minute, severe respiratory distress or oxygen saturation measured by pulse oximetry (SpO2) < 90% breathing ambient air at rest, and critical pneumonia in pa tients with respiratory insufficiency who required mechanical ventilation15. All were measured with spirometry before and after bronchodilators, and the diffusing capacity for carbon monoxide (DLCO) was measured following the American Thoracic Society/ European Respiratory Society (ATS/ERS) guidelines using a Platinum Elite TM Series Body Plethysmo graph (Medical Graphics Corporation, Minnesota, USA)16,17.
The results were interpreted according to the guidelines performed by the leading societies and experts18-20. A chest CT scan was made on the patients at the moment of the pulmonary function evaluation. The method employed by Michael et al was used to quantify the severity of the lung involvement (total severity score)21. Each of the five lung lobes was as sessed for degree of involvement and classified as none (0%), minimal (1-25%), mild (26-50%), moderate (51-75%), or severe (76-100%). Each of these degrees was correlated with a score of 0,1,2,3 and 4, respectively, with a range of possible scores from 0 to 20. Apart from the “total severity score”, the pres ence of the following characteristics was evaluated: ground-glass opacities, consolidation, fibrosis, honeycombing, crazy paving, air trapping, pleural effusion and lymphadenopathies. The presence of dyspnoea was evaluated at the moment of the medical consultation. Age, sex, anthropometrical data, smoking history and past illnesses were registered.
The continuous variables were described using the media and standard deviation or median and interquartile range followed by the Student T- test for unpaired data or Mann- Whitney according to their distribution. Categorical variables were described as a percentage and were compared using the chi-square test. The association between two quantitative continuous variables was made using Pearson correlation analysis. The statistical analysis was made with Infostat/L 2016 version.
Informed written consent of all patients was obtained and approved by the ethical committee of the Hospital A. Posadas.
Results
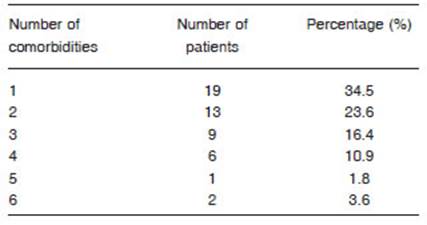
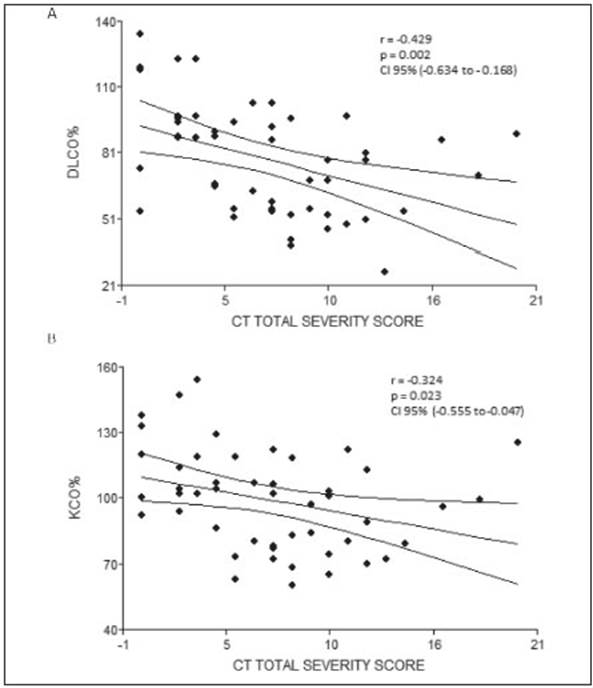
Fifty-five patients were included, 40 males (73%), whose media and standard deviation age was 54.9 (11.6) years old with a body mass index (BMI) of 33.1 (6.1) kg/m2. Fifty (90%) had at least 1 comorbidity, being the most frequent obesity 67%, arterial hypertension 36% and diabetes mel litus 35% (Tables 1 and 2). Of the 55 evaluated 25 (45%) had critical pneumonia with requirement of mechanical ventilation (MV), the median days in MV was 17 (10-22), 30 had severe pneumonia, 21 (38%) required oxygen therapy with a reservoir bag and only 9 (16%) were treated with a nasal cannula. The average pulmonary function measured by spirometry of the patients who had severe or critical COVID-19 pneumonia was normal and the DLCO was slightly under 80% (Table 3). When analyzing the spirometries 15 (27.3%) showed a ventilatory defect that suggested restriction, while the remaining 40 spirometries (72.7%) were normal. No obstructive disturbance or positive bronchodilator response was registered. The evaluation of the gas exchange using DLCO revealed that 28 patients (51%) had values under 80%. When the analysis was made considering the values of DLCO, carbon monoxide transfer coefficient (KCO) and alveolar volume (VA) a gas exchange disorder was registered in 32 (58%). No significant differ ences were found when comparing the values of pulmonary function between patients who did not require MV (severe pneumonia) and those who did (critical pneumonia) (Table 4). Patients who had a gas exchange defect had forced vital capacity (FVC), forced expiratory volume in the first second (FEV1) and DLCO values significantly lower than those who did not have this defect (Table 5). No significant differences were found in age 53.3 vs. 56 years (p 0.40), BMI 33.6 vs. 32.8 kg/m2 (p 0.64), male gender 73.9% vs. 71.9% (p 0.86), comorbidities or MV requirement between patients who had a gas exchange defect and those who did not (Table 6). The image analysis was made in 49 of 55 scans. Ninety percent had some degree of involvement in the chest CT scan. The media “total severity score” was 6.8 (± 4.7). The most common findings were the presence of ground glass opacities in 40 (82%) patients and reticular linear and thick opacities in 35 (71%). With less frequency air trapping was observed in 7 (14%), lymphadenopathies 3 (6%), consolidation 2 (4%), honeycombing 2 (4%) and pleural effusion 1 (2%). A significant difference was found in the “total severity score” between patients with a gas exchange defect and those without it 8.2 (± 4.1) vs. 5.1 (± 5.0) p = 0.02. This did not occur when comparing the same score between patients who did or did not refer dyspnoea, or between those who required or not MV, 6.6 (± 4.2) vs. 7 (± 5.4) p = 0.81 and 6.9 (± 4.7) vs. 6.7 (± 4.9) p = 0.92. A moderate negative correlation was found be tween the severity of the tomographic involvement and the DLCO r = -0.429 (CI 95 -0.634 to -0.168) p = 0.002 and KCO r = -0.324 (CI 95 -0.555 to -0.047) p = 0.023 (Fig. 1). At the time of consultation 30 (55%) referred some degree of dyspnoea. Patients with this symptom had DLCO and KCO values significantly lower than those who did not have dyspnoea 70.5 vs. 85.1 p = 0.02 and 88 vs. 104 p = 0.02 respectively.
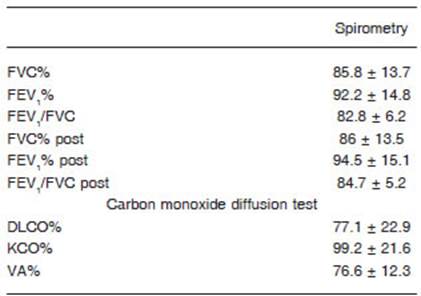
Table 3 Average spirometry and carbon monoxide diffusing capacity values of patients who had severe or critical COVID 19 pneumonia 3 months after hospitalization

Table 4 Comparison of spirometry and carbon monoxide diffusion capacity values between patients who had a severe and critical pneumonia

Table 5 Comparison of spirometry and carbon monoxide diffusion capacity between patients with and without a gas exchange defect (DLCO and KCO values below the expected for the measured VA)

Table 6 Differences in comorbidities and mechanical ventilation requirement between patients with gas exchange defect and those without it
Discussion
As COVID-19 represents a new disease little is known about the pulmonary results in the long term in survivors of severe forms of the illness. The patients in our study, all of them with severe or critical disease, had an average age of 55 years old with a prevalence of men, similar to what was found in recent studies where the media of age with severe or critical pneumonia varied between 53 and 61 years old22-24. Ninety percent presented at least one comorbidity, being the most frequent obesity, diabetes mellitus and arterial hypertension. While these appear in all series as the most frequent comorbidities, in our cohort the main comorbidity was obesity (67%). It is remarkable that in the published studies of pulmonary function and COVID-19 obesity is not included as a frequent comor bidity. In the initial publications, with less severe patients and without critical ones, the BMI varied between 23 and 25 kg/m² (within the normal range)12,13. In recent studies with a bigger number of severe patients the BMI was slightly higher but in the overweight range, 25.8 to 29.8 kg/m2, and only in the study published by Bellan M et al, although the BMI is not mentioned, obesity appears as comorbidity present in 10.5%22-24.
The impact on lung function in the different severity degrees of COVID-19 is poorly documented. After an observation time of 3 months, our cohort of patients with severe or critical COVID-19 pneumonia presented on average a normal pulmonary function measured by spirometry, with a slight drop of the DLCO values under 80%. These findings were similar to those found in papers with patients that had both a mild to moderate disease as in those who suffered a severe or critical illness10-13,22-24. Nevertheless, when analyzing separately the values of the pulmonary function of patients with a gas exchange defect and those without it, the differences are very significant. It is important to highlight that 58% of patients presented a gas exchange defect and 27% low values of FVC consis tent with restriction, data that is lost or unobserved when only analyzing the average values. When comparing with numbers available in the bibliography, the percentage of patients with FVC values under the normal range varies between 7 and 11%, while the DLCO is affected in 16 to 84% according to the different series11-14,22-24. The rate of radiological abnormalities present in our study was within the highest, 90% had some involvement in the chest CT scan, with a strong predominance of ground-glass and reticular opacities. Only 2 patients had honeycombing. It is important to mention that none of them had a history of known interstitial lung disease. Two of the 7 patients with air trapping were former smokers and 1 had a history of smoking and asthma. In our study we found a moderate correlation between the severity of image lung involve ment and DLCO and KCO levels, this implies that other mechanisms in addition to parenchymal damage could be responsible for the gas exchange defect. More than half referred dyspnoea and had lower DLCO and KCO values than those who did not have this symptom, what makes the gas exchange defect a possible cause of it.
The presence of abnormal gas exchange is the main characteristic of the patients with COVID-19 sequelae, this is reflected by the DLCO and KCO values which are under the expected ones for the alveolar volume (VA) in which they were measured19,20. We cannot determine with our study´s data whether this is due to the rupture of the alveolar-capillary barrier or the abnormal pulmonary blood volume. The interstitial lung disease associated with the acute respiratory distress syndrome in patients with CO VID-19 probably harms the alveolar- capillary units. This would lead to a gas exchange defect through the loss of these units. The result would be a reduction in the DLCO, KCO and VA25. Also, an increasing suggestion of pulmo nary hemodynamics alteration in patients with COVID-19, including the loss of the vascular bed with a decreased pulmonary blood volume measured by high resolution computed tomography26,27. This would reduce the DLCO and KCO, but in this case, the VA would be preserved. In our study the restrictive defect suggested by a low FVC in only 27% does not completely explain the abnormal val ues of DLCO and KCO present in 58%; what is more the marked fall in these values does not correspond with the mild fall in the FVC, this indicates that another mechanism such as the alteration of the pulmonary hemodynamics formerly described could be involved.
Even though the patients with severe and critical pneumonia assessed by us between the 8th and 12th week after diagnosis did not have significant abnormalities in the pulmonary mechanics as evaluated by spirometry, the presence of dyspnoea and a high total severity score in the chest CT scan reflect gas exchange anomalies observed in the DLCO tests. The analysis of our study does not arise predictors of evolution towards pulmonary sequelae since we did not find an association between the gas exchange defect and the presence of comorbidities or the severity of the course of the disease. The existence of other fac tors inherent to the patient or the disease that were not analyzed or that we do not know would be involved in the evolution of COVID-19 towards pulmonary sequelae.