KEY POINTS
• Several therapeutic alternatives have been developed for the treatment of severe COVID-19 pneumonia. TCZ combined with dexamethasone decreases mortality in patients who require oxygen supply.
• In this cohort, obesity and CRP values do not seem to be associated with a higher risk of superinfection or death. The absence of invasive mechanical ventilation, shock and superinfection resulted in better prognosis.
Since the initial description on December 31, 2019, in Wuhan City, Hubei province (China); of an acute respira tory disease1 caused by a new coronavirus (SARS-CoV-2) and its spread worldwide, multiple, and different treatment approaches have been studied.
Among these therapeutic interventions, use of systemic steroids showed clear reduction in mortality in hospital ized patients with Coronavirus disease (COVID-19) that required oxygen supply2.
Tocilizumab (TCZ) is a humanized recombinant monoclonal antibody against interleukin-6 (IL-6) receptor, that inhibits the binding of IL-6 cytokine. IL-6 is involved in inflammation, and it regulates the immune response. National Administration of Medicines, Food and Medical Technology (ANMAT)3, United States Food and Drug Administration (FDA) and European Medicines Agency (EMA) have authorized its use for treatment of rheuma tological illnesses (rheumatoid arthritis, juvenile idiopathic arthritis, giant cell arteritis, and polyarticular juvenile idio pathic arthritis). The hypothesis of a potential use of TCZ in severe COVID-19 patients is linked to the fact that the virus induces cytokine release (especially IL-6) in those patients with severe disease4.
TCZ most common side effects include headache and hypertension, and rarely, hepatotoxicity, that ranges from mildly elevated transaminases to severe drug-induced liver injury (DILI)5. Currently, data on TCZ hepatotoxicity in COVID-19 patients are limited and inconclusive6.
Despite the potential risk for secondary infections reported by some authors when using IL-6 pathway inhibitors, there are many randomized clinical trials that demonstrated there is no such higher risk7,8. The RE COVERY study9 showed that TCZ reduced the 28-day mortality in SARS-CoV-2 pneumonia patients that required oxygen support of any type and had C-reactive protein (CRP) levels above 75 mg/l. Besides, the REMAP-CAP10 database showed that TCZ reduced hospital mortality in patients who had started high-flow oxygen or more inten sive respiratory support in the previous 24 hours.
In our institution, we considered current evidence and used the National Institute of Health (NIH, United States) recommendations11 and the American Society of Infectious Diseases (IDSA) guidelines12 for treatment of COVID-19 patients. The aim of this study was to describe clinical and laboratory characteristics and outcome of patients receiv ing TCZ and systemic steroids as treatment for severe or critical SARS-CoV2 pneumonia in Sanatorio Finochietto in Buenos Aires City.
Materials and methods
We conducted a retrospective observational study based on electronic records of patients 18 years of age or older with severe or critical pneumonia due to SARS-CoV-2, who received treatment with TCZ (8 mg/kg/dose) and systemic steroids (dexamethasone ≥ 6 mg/day or another steroid at equivalent doses) from March to May 2021. Demographic and clinical data were gathered. The study took place at Sanatorio Finochietto, a private health center that provides health care services in prevalent clinical and surgical patholo gies. It is a reference center in Buenos Aires City, which has an Emergency Department, Maternity Ward, General Ward, Intensive Care Unit; as well as complementary services, such as laboratory, radiology, and hemodynamics departments. The protocol was approved by the institution’s Ethics Committee.
In the descriptive analysis, quantitative data was expressed as median and interquartile range 25-75 (IQR) according to their distribution. Data normality was evaluated using charts and Kolmogorov-Smirnov’s test. Qualitative data was ex pressed as absolute and relative frequency in percentage. For comparison between groups, chi2 or Fisher test were used according to assumptions for qualitative data and Wilcoxon for quantitative data according to their distribution. The Wilcoxon signed-rank test, a non-parametric statistical hypothesis test was used to compare CRP levels between TCZ and death or discharge. A significance level of less than 0.05 was consid ered. R software version 4.0.3 was used.
Results
The first 31 severe COVID-19 patients receiving TCZ treatment were included in the analysis. A patient was later excluded because he was referred to another facility within 24 hours of TCZ administration and due to inability to obtain data. Thus, 30 patients were finally included in the analysis. Median age was 56.5 (IQR 45.0- 66.2) and 19 (63.3%) were men.
Figure 1 shows patient’s concomitant morbidities.
All patients were admitted to the Intensive Care Unit. Median APACHE at admission was 8.0 (IQR 6.0-9.7). Quantitative biomarkers (CRP, ferritin and D-dimer) were evaluated at the time of TCZ administration (Fig. 2).

Fig. 2 Biomarkers at the time of TCZ administration. A) C-reactive protein (mg/dl) B) Ferritin (ng/ml) C) D-dimer (ug/ml)
Median number of days between COVID-19 symptoms onset and TCZ administration was 10.0 days (IQR 7.25- 11.0). All patients received systemic steroids; the median number of days was 14.0 (IQR 10.0-20.7). Five patients (16.7%) also received methylprednisolone pulses. Only one received convalescent plasma. All of them received only one TCZ infusion, and the dose did not exceed 800 mg.
Only two patients did not require ventilatory support be yond a non-rebreather mask. Of the remaining 28 (93.3%), twenty (66.6%) required high-flow nasal oxygen (HFNO) and 17 (56.7%) required invasive mechanical ventilation (IMV). A total of 11 patients required both HFNO and IMV due to worsening in the ventilation parameters. These patients and those who required IMV from admission were included in the IMV group. Figure 3 shows the days required for each type of ventilatory support.

Fig. 3 Days of requirement for each type of ventilatory support. A) High nasal flow oxygen B) Invasive mechanical ventilation
Median length of hospitalization was 17.0 days (IQR 13.0-23.7) and 14 patients (46.7%) presented secondary infections. When comparing patients ac cording to whether they had secondary infections or not, the former had a higher frequency of shock, multiorgan failure, IMV requirement and total length of hospitalization (Table 1).
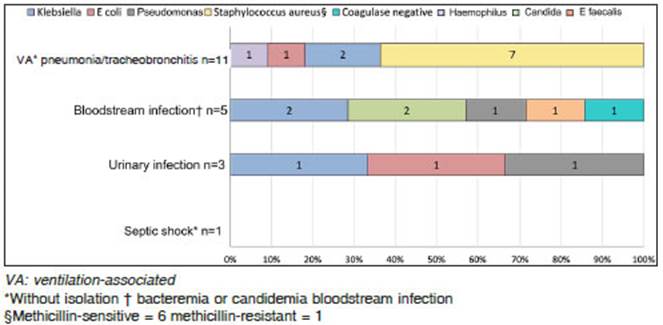
There were 22 secondary infections, and 6 patients had more than one event. Most of them were ventilation-related pneumonia or tracheobronchitis (11, 50%). Secondary infections and microorganisms found are described in Figure 4.
CRP levels decreased from the administration of TZC to death or discharge (Fig. 5 A). Seven patients died (23.3%). Compared to those who survived, they had a lower decrease in CRP levels between TCZ administra tion and discharge or death (Fig. 5 B). There was no association between obesity and death, this frequency was 3/7 (42/9%) in patients that didn’t survive and 10/23 (43.5%) in those who survived (p = 0.999). All patients who died required IMV (100%) versus 10 in those who survived (43.5%, p = 0.01). Shock, multi-organ failure and secondary infections were more frequent in those patients (in percent) 6 versus 2 (p < 0.001); 4 versus 1 (p = 0.006) and 7 versus 8 (p = 0.006), respectively. Neutrophil counts were normal. Elevated liver enzymes (five times above normal range) were only observed in two patients.

Fig. 5 A) Measurement of C-reactive protein levels(mg/dl) at TCZ treatment and at death or discharge
Discussion
This cohort represents a relatively young group of pa tients with severe SARS-CoV2 pneumonia, with an aver age age that fall below the age reported in Argentina for patients admitted to multiple intensive care units (UCI)13-15 and, with a prevalence of males in a 2:1 ratio compared to females. Interestingly, one out of three patients evaluated didn’t present any of the known risk factors for severe COVID-19. This may be related to the patient’s younger age. We did not find previous publications in Argentina of a cohort of consecutive severe COVID-19 patients who received TCZ treatment associated with standards of care.
Obesity found in our study population is similar than that reported in a recent publication of ventilated patients in ICU in Argentina in 202013, being this the most frequent concomitant morbidity in our series. Hypertension, which has the highest prevalence in Argentine publications13-15 came second. The same was reported for patients re ceiving TCZ as treatment for SARS-CoV-2 pneumonia in Chile16 in 2020, and Barcelona17 in 2021.
TCZ was infused in all patients within 24 hours of clinical worsening, and in most cases in the hyper inflam matory phase, eight patients (26.6%) received TCZ within the first 7 days of symptoms onset. All patients received parenteral corticosteroids and had a CRP value greater than 75 mg/dl, thus, standard indications suggested by RECOVERY were accomplished.
Our series shows that at TCZ administration, all pa tients had biomarker levels compatible with an augmented inflammatory response (CRP and ferritin). Unlike other publications16-18, none of them showed elevated D-dimer values.
Half of our patients developed secondary infections during hospitalization, most of them caused by bacteria. Ventilator-associated pneumonia (VAP) and methicillin-susceptible Staphylococcus aureus (MSSA) were the most prevalent infections and bacteria found, respectively.
Although IMV was related with an increase of sec ondary infections (p < 0.001), there was no association between IMV time and infection. Neither there was any association between secondary infections and CRP or ferritin values at clinical worsening.
We found an increase in mortality in patients that pre sented IMV requirements, shock, multiorgan dysfunction and secondary infection, and CRP values had a lesser decrease from TCZ to death. Although obesity is widely reported as a risk factor for bad outcomes for COVID-19 patients, in our cohort it does not seem to be a risk factor for secondary infections nor it seems to be associated with an increased mortality. Remarkably, Estenssoro et al13 found that the body mass index was higher in patients without secondary infections compared to those who survived.
Use of TCZ was safe and there were no adverse events during infusion and no need for discontinuation. No subsequent development of neutropenia was found. Elevated liver enzymes (five times above normal range)16 was only observed in two patients (6.7%) and this was lower to that reported in registry trials (10-40%)6. None of them had liver failure.
Our study has several limitations. It is a single center, observational study, with no control group and with a small number of patients. Long-term survival and patient’s condi tion after hospital discharge was not assessed. Despite this, the strength of this study relies on the fact that it is an Ar gentine experience of the use of TCZ for treatment of severe COVID-19 patients, with a survival rate greater than 75%.
Neither obesity nor CRP values at clinical worsening seem to be risk factors for higher chance of secondary infections or death, although more studies are needed to draw further conclusions.