KEY POINTS
Current knowledge
• Multimodal ultrasonography is a useful tool for the man agement of the critically ill patient at the bedside, al though it is time consuming and not always possible in critical care. Limited information is available on echocar diographic findings in critically ill patients with mechani cal ventilation and acute respiratory distress syndrome secondary to COVID-19.
Contribution to current knowledge
• In this series, the main echocardiographic findings were right ventricular dilatation, pulmonary hypertension and new or dynamic regional left ventricular contractility disorders.
Coronavirus Disease 2019 (COVID-19) produces a significant burden to severely ill patients affected by acute respiratory failure. Beside lung injury, it has been described in this setting endothelial damage, throm boembolic disease and cardiac dysfunction. However, systematic evaluations of these other potential problems are lacking.
Bedside ultrasound is a valuable tool for critical care patient’s management1-4. During last year, multi-organ point-of-care ultrasound including lung ultrasound and focused cardiac ultrasound as a clinical adjunct has played a significant role in triage, diagnosis and medical management of COVID-19 patients5. Echocardiography and vascular Doppler are useful for non-invasive hemo dynamic status interpretation and monitoring6-8. They provide different Doppler and bidimensional measures for hemodynamic parameters and key aspects for the interpretation of hemodynamic instability and respiratory failure9,10. These systematic assessments in critical care subjects might improve patient care and prognosis11.
The aim of this study was to describe echocardio graphic findings in a series of mechanically ventilated patients with moderate and severe ARDS (acute respira tory distress syndrome) due to COVID-19.
Materials and methods
This was a single center, descriptive and cross-sectional study of prospectively collected data.
All patients admitted between June 1 and December 31 2020, to the ICU (intensive care unit) of our center were screened for eligibility. We included patients with laboratory-confirmed Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection defined by a positive result of real-time reverse transcriptase-polymerase chain reaction assay of nasal or pharyngeal swabs, and moderate or severe ARDS as defined by the Berlin criteria12.
Exclusion criteria were age <18 years and patients with withhold life sustaining measures.
Clinical management (including mechanical ventilation settings and pharmacological therapies) followed our center treatment guidelines.
An electronic case report form (REDCap electronic data capture tools13) hosted at Instituto Universitario CEMIC was used for data collection. Information regarding demographic, anthropometric and clinical data (comorbidities14, severity scores15,16, vital signs, type of respiratory support, respiratory parameters, laboratory tests including blood gas analysis) were collected on the day of admission to the ICU.
Following inclusion, an initial echocardiogram was per formed within 7 days of ICU admission and every 15 days afterwards until mechanical ventilation ended, 28 days or death. Additional echocardiograms were performed on an “as needed” basis, according to the attending ICU physician. All echocardiographic assessments were performed by a single physician with extensive experience. Each evaluation was recorded for a second validation by another expert. Time spent by the physician for each study was measured. The following echographic measurements were acquired: left ventricle (LV) ejection fraction (Simpson’s method or eyeball assessment by two independent trained physicians) (LVEF); LV hypercontractility was defined by a LVEF greater than 80% with low LV end systolic volume or diameter;LV regional wall abnormali ties (assessed by two independent trained physicians); Mitral Doppler: E peak velocity and E/A ratio; Tissue Doppler: septal e’ peak velocity and E/e’ ratio; LV outflow tract velocity time integral (VTI); Right ventricle outflow tract acceleration time (pulmonary hypertension was considered when acceleration time was lower than 85 ms); Estimated systolic pulmonary pressure (PsP) by tricuspid regurgitation peak velocity and central venous pressure (measured by central catheter when available, or estimated by inferior vena cava visualization). Pulmonary hypertension was considered when the estimated PsP was greater than 40 mmHg; Right ventricle (RV) diam eter (measured from subcostal 4 chamber view and apical 4 chamber view; mild RV enlargement was defined as diameter greater than 75% of LV diameter and moderate/severe RV enlargement when it was greater or equal to 100%); Tricuspid annular plane systolic excursion (TAPSE; RV systolic dysfunc tion was defined when TAPSE was lower 17 mm)
A diagnostic interpretation or conclusion was also provided by the performing physician, when chronic or acute significant changes were found in the study.
Transthoracic echocardiography is not always possible in critical care patients due to difficulty in achieving acceptable images from classic transthoracic views (left parasternal, api cal and subxiphoid). In addition to this, when feasible, it may require a long time to perform. We defined three categories for possible study outcomes, dividing them into adequate, suboptimal and inadequate. All inadequate studies were not considered for analysis and only suboptimal and adequate studies were used for final analysis. A study was defined as adequate when 2 or more of the classic views offered ac ceptable images and more than 70% of the 10 prespecified measures could be correctly achieved. The study was subop timal when only one of the classic views offered acceptable images or less than 70% of prespecified measures could be achieved. Finally, it was considered inadequate when none of the classic views offered acceptable images or less than 40% of pre-specified measures could be achieved.
Results
Forty-five patients fulfilled the inclusion criteria. After ex clusions (4 patients who were never intubated, 2 patients with withhold of life sustaining measures and 6 patients with inadequate echocardiographic acoustic windows), 33 patients were analyzed. From these 33 patients, 7 (21%) had only the initial study available for analysis, and the rest underwent echocardiographic follow up until day 28 (Fig. 1).

Fig. 1 Flow chart in patients under mechanical ventilation with COVID-19 acute coronary distress syndrome
Continuous variables are presented as median and interquartile range [IQR]. Categorical variables are ex pressed as frequencies (percentages).
The total number of echocardiograms performed was 76, of which 48 (64%) had an adequate acoustic window while in the remaining 28 (36%) the acoustic window was suboptimal. The median imaging time required to complete a standard study in this setting was 13 [10-15] minutes. It should be noted that this time measurement does not include the time required for personal protective equipment donning and doffing as well as the time taken to clean the equipment.
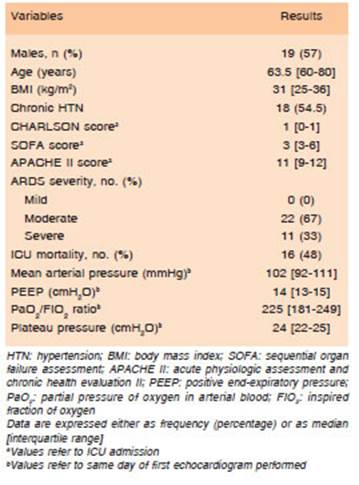
Table 1 summarizes the patients’ demographic and clinical characteristics at ICU admission and their clinical outcomes. 57% were male and median age was 63.5 [59.5-79.5] y/o. 54.5% had chronic arterial hypertension and median body mass index was 31 [25-36] kg/m2. Median SOFA and APACHE II scores at ICU admission were 3 [3-6] and 11 [9-12], respectively. ARDS severity was moderate in 69% and severe in 31% of the cases. Mortality at day 28 of follow-up was 48% (16 patients).
Chronic structural abnormalities were present in 16 of the 33 patients included for analysis (48.5%). The main finding was mild LV myocardial hypertrophy in 11 patients. Other findings were left atrial enlargement (mild in 5 patients and moderate or severe in 2 patients), aortic valve regurgitation (3 patients), mitral valve regurgitation (5 patients) and 1 patient with mild aortic valve stenosis.
Table 2 summarizes main findings of the echocardio gram performed at admission.
At the initial echocardiographic assessment, RV dilation was present in 13 patients (39%): 12 of them had mild RV enlargement, while the remaining had moderate/severe RV enlargement. When all 76 echocardiograms were included, RV dilation was present in 33 studies (43%): 4 of them had moderate/severe RV dilation and the remain ing mild. Other findings were: pulmonary hypertension (5 patients - 15%); new or dynamic LV regional wall motion abnormalities (5 patients - 15%), including 3 patients with inverted Takotsubo like image; new or dynamic LV global contractility deterioration (2 patients - 6%); hypercontrac tility present in 4 patients (12%) (usually associated with tachycardia); finally, 1 patient with atrial enlargement that had no thrombotic complications in the first echocardio gram developed a new large left atrial clot that generated LV inflow tract obstruction with consequent irreversible shock during his follow up.
All 7 patients with regional or global wall motion abnor malities had complete recovery within 28-day follow-up.
One of the patients with a Takotsubo like pattern in the initial echocardiogram, had 2 more Takotsubo events associated with septic complications during the follow-up. This patient underwent a final echocardiographic assess ment at 60 days and showed a total recovery.
From the 7 studies with a LV outflow tract VTI less than 17 cm, 3 had systolic dysfunction with Takotsubo pattern, 1 had systolic dysfunction with an acute regional septal and anterior contractility disorder, 2 had hypovolemic
status (both patients with E peak velocity below 60cm/s, E/A ratio ≤ 1, E/e´≤ 10, LVEF ≥ 65% and low end systolic volume) and 1 corresponded to the previously mentioned left atrial thrombosis with obstructive shock.
Discussion
RV enlargement, pulmonary hypertension and new or dynamic LV global and regional contractility abnormalities were our main findings.
Transthoracic echocardiography is not always possible in critical patients with ARDS under mechanical ventilation. This may be influenced by patient position (prone ventila tion), patient’s subcutaneous edema, high positive end expiratory pressure with pulmonary areas of hyperinflation and patient’s thoracic anatomy. When plausible, it may be time consuming. Even so, the method is useful in most patients and brings reliable information, sometimes with immediate therapeutic implications.
The most frequent finding was RV enlargement (43%) considering not only the initial assessment, but all echo cardiograms from the 28-day follow-up for each patient. The known association between this and mechanical ventilation has been well described by Vieillard-Baron and colleagues17. While Vieillard´s assessment was made by a LV end diastolic diameter to RV end diastolic diameter ra tio greater than 0.6, our RV dilation definition only included those with a ratio greater than 0.75 (an index of 0.6 would have overestimated our results). We did not contemplate the septal motion in a short axis for RV dilation diagnosis (although an important physiological finding for a better interpretation, it was not considered in our setting). RV enlargement higher incidence could be explained by a high level of PEEP used in our patients (median 14 cmH2O) although plateau pressure was always less than 30 cmH2O (same parameter used by Vieillard for defining protec tive ventilation). Furthermore, this finding could also be associated with hemodynamic or thrombotic changes in pulmonary circulation generated by COVID-19 ARDS and hypoxemia. However, every patient with dilated RV and/ or elevated PSAP underwent a simultaneous venous dop pler of the lower limbs: no thrombotic events were found.
LV global and regional wall motion abnormalities, including Takotsubo, have also been well described in critically ill patients, especially in sepsis18. This could be explained in our series by the presence of Systemic Inflammatory Response Syndrome (SIRS) generated by COVID-19 and the consequent stress response. 1 of the 3 patients with TakoTsubo at the initial echocardiogram showed a total recovery by the second week assessment. However, during 2 posterior septic events within his ICU stay, myocardial involvement was reproduced showing the high risk of these patients to repeat contractility deteriora tion when re-exposed to stress.
Only 1 confirmed thrombotic complication was found in our series. It is noteworthy that all patients included received high dose chemoprophylactic with low weight heparin (1 mg/kg of enoxaparin) from day 1 of ICU admission.
Another frequent finding was hypercontractility with low end systolic left ventricular volume. This may be due to restrictive fluid management strategies in ARDS patients, to the presence of SIRS or to the development of tachycardiomyopathy or arrhythmic complications (the latter was not evaluated in our study).
Among chronic structural abnormalities, mild LV myocardial hypertrophy was the main finding. This may respond to the high prevalence of chronic arterial hyper tension in our population (54.5%).
In conclusion, transthoracic echocardiography is not always plausible in critical patients with ARDS under mechanical ventilation. However, it continues to prove its usefulness in most patients and brings reliable informa tion, sometimes with immediate therapeutic implications. Although echocardiography is available bedside and with no harm or risk for the patient, it has proven to be time consuming for the attending physician.