The use of radiation plays an important role in the management of pituitary adenomas and may be consid ered at any point of its treatment algorithm1,2. As adjuvant modality, it has been used in patients with functioning or non-functioning adenomas with remaining or recurrent tumor after surgery, achieving long-term tumor control in 80-97% of patients and endocrine remission in 40-70% of cases. The radiation dose necessary to stop tumor growth (antiproliferative effect) is lower than the dose necessary to achieve normalization of hormonal hypersecretion1, Most authors report a radiosurgical median margin dose of 22 Gy (range 14-35) for GH-secreting adenomas and 24 Gy (range 15-35) for ACTH-secreting adenomas and lower doses for nonfunctioning pituitary adenoma (NFA)1,3. Among adverse effects of radiation treatments, hypopituitarism occurs in 30-60% of patients 5 to 10 years after irradiation, while radio-induced optical neuropathy, strokes and second tumors have been reported in 0-3% of the cases4,5. Stereotactic radiosurgery delivers a single high concentrated dose of radiation to the target, with a steep dose falloff, relatively spearing radiation exposure to surrounded normal tissue. Nowadays, stereotactic radiosurgery, and particularly gamma knife (GKS), is the most frequently used adjuvant technique in patients with recurrent or persistent pituitary adenomas. It can also be considered as an upfront treatment if there is a medi cal contraindication for neurosurgery, if the outcome of neurosurgery is uncertain considering the unfavorable position of the adenoma (cavernous sinus) or if the patient refuses the neurosurgery6,7. Clinical work with the GKS began in 1967 in Sweden. Units 3 and 4 were placed in Buenos Aires in the early 1980’s. FLENI has the only GKS equipment available in Argentina. The main purpose of this study was to describe the national experience with GKS for pituitary adenoma patient´s management.
Materials and methods
We conducted a retrospective analysis of patients with pitu itary adenomas treated with GKS between 2002 and 2017 in FLENI, Buenos Aires, Argentina. Adult patients with NFA, acromegaly, Cushing’s disease, prolactinoma and mixed pituitary tumors were included: Among patients with ANF, those with post-operative tumor remnant who presented parameters of risk of regrowth due to tumor invasion of the cavernous sinus and / or proliferation index (ki67) ≥ 3% or who had evidence of regrowth were included. Patients with Cushing’s disease included were those with tumor remnant and active disease in the postoperative period. Among the acromegalic patients, the majority were also those with residual tumor and active post-surgical disease. In this case, unlike Cushing’s disease, given the availability of medical treatments that allow adequate long-term clinical control, we recommended GKS mainly in those cases whose target conformation allowed to minimize the collateral radiation to the normal pituitary gland and the hypothalamus. The series also included some treatment-naive patients (without previous surgery) in cases in which the involvement of cavernous sinus predicted non-postoperative remission or in patients with contraindication or refusal to surgery. Patients with less than one year’s follow-up or be ing managed by endocrinologists from other institutions were excluded. Every effort was made to limit the radiation dose to pituitary stalk to <15Gy and to the optic apparatus to < 8 Gy. So, patients with tumors whose proximity to the optic chiasm was risky did not receive GKS and were also excluded.
Radiosurgical treatments were delivered in a single day session with the Leksell Gamma Knife (Elekta Instrument AB): model B (n:67), Model 4 (n:21) or PerfeXion (n:11). All procedures were performed by the team of FLENI’s radiosurgical unit. According to the usual practice in referral centers, the pre scription tumor dose was selected depending on tumor type, being usually lower in NFA and higher in Cushing disease.
The negative relationship between hormone suppressive medications given at the time of radiosurgery and outcome following the procedure has been reported8-11. As a result of these studies, we discontinued antisecreting medications for 6 weeks before GKS, and resumed them 2-6 weeks after it.
The following aspects were evaluated: epidemiological and clinical data at diagnosis, target volume, radiation dose delivered to the tumor and to the normal pituitary gland, biochemical remission rate (for functional tumors) and tumor control rate, time to remission and treatment´s adverse effects.
Endocrine testing was performed in different laboratories. Therefore, the reference ranges used may vary. The endo crine biochemical remission was defined as the normaliza tion of 24-hours urine free cortisol (UFC) levels in Cushing’s disease, prolactin in prolactinomas, thyroid hormones in thy rotropinomas and IGF-1 (with growth hormone level < 2.5ng/ ml) in acromegaly12. Only a few patients had an oral glucose tolerance test (OGTT), so we used a less strict criteria to define acromegaly remission. Efficacy was assessed while patients were off medical treatment for 3 months in all cases. After GKS, a thin-slice pituitary adenoma protocol using 1.5 or 3 Tesla MRI was performed to detect changes in tumor size, biannually in the initial year and subsequently the radiographic study was obtained yearly. Tumor control was defined as either no demonstrable growth or shrinkage (more than 20%) of the adenoma on follow-up imaging13. New onset of hypopituitarism was defined as a pituitary hormone deficit after GKS, requir ing corresponding hormone replacement (that was started as soon as the deficiency was detected). Specifically, given the fact that for a long time and even now we have not had avail ability to perform the corticotropin test, corticotroph deficiency was defined as low morning serum cortisol and a normal or low serum ACTH level5. Thyrotroph deficiency was based on a low free T4 with normal or decreased thyroid-stimulating hormone. Gonadotroph deficiency was defined as low or nor mal gonadotropin levels and low plasma testosterone in men and amenorrhea with low plasma estradiol in premenopausal women (< 50 years old) and low gonadotroph levels in post-menopausal women14-16. GH deficiency was not included in the analysis because age- and sex-adjusted IGF-1 was available only in a few patients. Each tumor subtype was analyzed as a group and findings were compared with the available literature.
We looked for independent variables that could predict tumor control, endocrine remission and the risk of developing hormone deficiencies. Specifically, we analyzed differentially the outcome of patients with adenomas with ki67 index ≥ 3%. Some studies reported a higher risk of tumor progression in irradiated patients more than 6 months after surgery vs. those who received GKS early17. So, we compared patients treated with ¨early GKS¨ in an adjuvant setting after incomplete resection (less than six months after surgery - true adjuvant) vs. those that received ¨late GKS¨ (progression or recurrent setting). We also divided acromegalic patients in two groups according to the pre-treatment IGF-1 levels (> or < 2 upper limit normal - ULN) to analyze if there is a correlation with GKS efficacy and IGF1 level.
For statistical analysis we used Stata version 12.1. We con sidered significance to any values of p < 0.05. Dichotomous variables were analyzed by means of Chi square or Fisher’s exact test. Continuous variables were analyzed by means of ANOVA or Kruskall Wallis test, depending on their distribution.
The study protocol was approved by the institute’s ethics committee (CEI) (approval number 04-17 / 06-17). As it was a retrospective study, the request for informed consent was waived.
Results
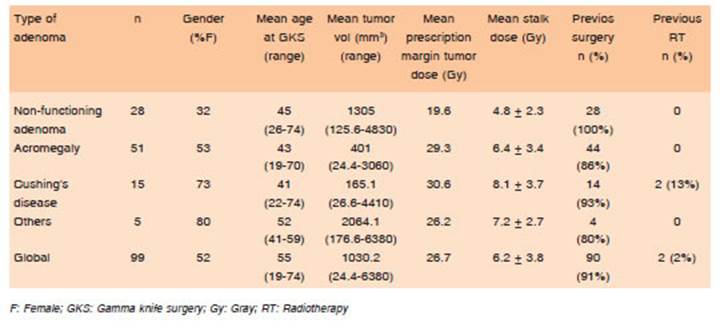
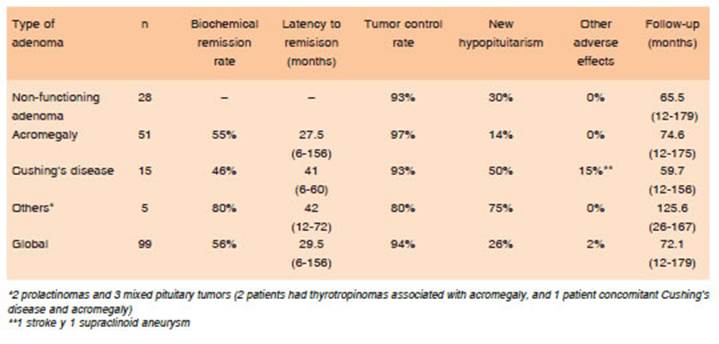
Ninety-nine patients with pituitary adenomas treated with GKS were included in the analysis. Of this cohort 51 were female and 48 males, with a mean age at the time of ra diosurgery of 45 years (range: 19-74): 51 had acromegaly, 28 NFA, 15 Cushing’s disease, 2 prolactinomas and 3 mixed pituitary tumors (2 patients had thyrotropinomas associated with acromegaly, and 1 concomitant Cushing’s disease and acromegaly). In 90 patients (91%), GKS was prescribed following incomplete surgical resection (mi croscopic or endoscopic transnasal surgery): 28 of them as an adjuvant therapy if a residual tumor was seen and in the remaining 62 during the follow-up, mostly in case of progression or recurrent setting. In 9 patients, radio surgery was the first line treatment. Two had undergone prior conventional radiotherapy. The mean prescribed margin dose was 30.6 Gy for corticotropinomas, 29.3 Gy for somatotropinomas and 19.6 Gy for non-secreting ad enomas (Table 1). The mean follow-up was 72.1 months (range: 12-179). Global tumor control rate was 94.2%. Biochemical remission rate was 55.9%, being higher in acromegaly than in Cushing’s disease (OR 4.7, 95% Ci 2.1-10.4, p < 0.0001). The mean time to remission was 29.5 months (range: 6-156) (Table 2).
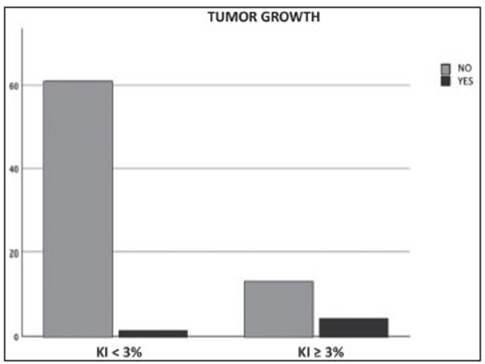
From the total population analyzed, 17.1% had Ki67 ≥ 3% (proliferative adenomas). The majority (53%) of them were NFA. During follow-up, tumor growth was seen in 23.5% of the proliferative adenomas and in 1.6% of patients in the group with Ki67<3% (p = 0.007) (Fig. 1). Among functioning adenomas, proliferation index was not significantly correlated with biochemical response at the end of follow-up (p = 0.147).
When we compared patients treated with early vs. late GKS, neither tumor control rate (92.6% vs. 94.2% respectively, p = 0.77) nor biochemical remission (52.9% vs. 54.7% respectively, p = 0.90) differed between both groups. No patient who received early GKS, with Ki67 < 3% had tumor growth (p = 0.043).
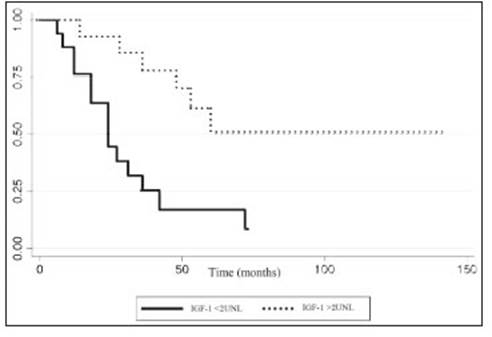
In acromegalic patients, we found that pre-GKS IGF-1 levels < 2 ULN were significantly associated with a higher remission rate (OR 0.99, 95% Ci 0.997-0.999, p = 0.009) (Fig. 2). No other biochemical remission or tumor control prognostic factor was found.
The most frequent adverse effect was hypopituitarism (26%). Patients with Cushing’s disease were more prone to develop any pituitary deficiency after GKS (OR 2.93, 95%Ci 1.2-7.2, p = 0.019). We did not find any correla tion between the dose to the normal pituitary gland/stalk and the development of hypopituitarism (OR 1.00003, 95%IC 1.0000004-1.00005, p = 0.021). One patient with Cushing’s disease, uncontrolled diabetes mellitus and previous radiotherapy suffered a thalamic stroke within 30 days after undergoing GKS treatment. Another patient, also diagnosed with Cushing’s disease, presented with a homolateral supraclinoid aneurysm 4 years after the ra diosurgical procedure. We did not find any case of visual changes or optic neuropathy after GKS.
Discussion
The population in our research is widely heterogeneous as we included patients with functioning and non-functioning pituitary adenomas with different tumor proliferation and invasiveness features, data which must be taken into con sideration when interpreting results. Patients with secret ing tumors were treated with significantly higher radiation doses than those with non-secreting adenomas, being even higher in those with Cushing’s disease (p < 0.0001). We observed a global biochemical remission rate of 55.9% after an average time of 2.5 years and a global tumor control rate of 94.2%, results which were similar in all groups and according with previously reported stud ies5-7,18. Furthermore, the tumor control rate was similar to that previously reported with the use of multisession stereotactic radiosurgery in our country19. We did not find significant differences in these rates in patients who underwent early vs late GKS, as have been observed in previous researches17. Accordingly with Sheehan et al20, in our acromegalic patients, lower pre-treatment IGF-1 levels were significantly associated with a higher remission rate. the same was reported in some surgical series21. The most frequent adverse effect was hypopituitarism, with a global rate of 26%, being higher in Cushing’s disease (50%) and lower in acromegaly (14.3%), with an average latency of 56.4 months after GKS. As growth hormone deficit was not assessed, hypopituitarism rates may be higher than noticed. No significant correlation was found between the radiation dose received by the remaining healthy pituitary gland/stalk and the subsequent development of hypopituitarism, as it was shown in previous research22. Even though visual impairment is frequently reported as side effect, it was not found in our patients, perhaps due to selection bias (we did not perform GKS if distance to optic chiasm was not safe).
In this paper we describe our experience of more than 15 years using GKS in patients with pituitary adenomas. In half of the patients with active, persistent, or recurrent disease with an identifiable tumor remnant, GKS was an effective option to achieve long term biochemical remis sion. This is essential in the management of patients with active Cushing’s disease or acromegaly, where control ling the hypersecretion is the main goal. GKS could also be considered for pharmacologically controlled patients with small remnants, far from the optical apparatus and under medical treatment to reduce chronic use of drugs and cost-minimization.
In our series, patients with Cushing’s disease were more prone to develop pituitary deficiency after GKS, while those with acromegaly showed lower rates of hypopituitarism. This difference could be explained by a bias in the selection of patients: in the case of Cushing’s disease, we prioritized the resolution of the hypersecre tory state over any possible adverse effect, while in those with acromegaly we recommended GKS mainly in those cases whose target conformation allowed to minimize the collateral radiation to the normal pituitary gland and the hypothalamus.
Concerning non-functioning adenomas, the 28 ana lyzed patients who had had previous surgery were prescribed GKS on clinical-pathological and radiological bases, as they showed high probability for recurrence and/or evidence of growth. For decision-making, we relied on our previous experience, in which we found a higher recurrence rate in patients with non-irradiated remnants of NFA with Ki-67 ≥ 3% in comparison with a significantly lower growth rate of those that had been irradiated after surgery23.
This study shows Argentine experience with the use of GKS in patients with pituitary adenomas, with similar re sults to those reported by centers with large radiosurgical practice5-7,18. In our experience, GKS achieved biochemical remission in more than 50% of patients and tumor control in most of them. Hypopituitarism was the most frequent adverse effect, while the occurrence of other adverse effects was infrequent. Stereotactic radiosurgery with gamma knife is an effective therapeutic tool for patients with pituitary adenoma following post-surgical recurrence, potentially aggressive persistence and/or resistance to pharmacological treatment.