KEY POINTS
• Lithium carbonate affects multiple organs such as kidney, heart, the motor endplate and thyroid and parathyroid glands.
• Thyroid disorders are the most common endocrine ad verse effects of lithium therapy; including hypothyroidism, hyperthyroidism, goiter and exophthalmos.
• Lithium-induced hypercalcemia and hyperparathyroidism may sometimes require surgical treatment.
• Nephrogenic diabetes insipidus generally remits with discontinuation of the drug, although chronic renal failure can occur.
• A baseline biochemical study and periodic monitoring should always be carried out after the beginning of lithium therapy to prevent major complications
Lithium carbonate is a psychotropic drug frequently prescribed as an antimaniac drug, in the treatment of aggressive-impulsive behaviors and mainly in bipolar disorders1. It is an inorganic salt formed by two lithium (Li+) cations and a carbonate anion (Li2CO3) (Fig. 1). It is also widely used in industry, such as manufacture of rechargeable batteries, electrical porcelain, coating of welding electrodes, glues, luminescent paints, varnishes and colorants2-4.
In 1970, thirty years after the first description by John Cade5,6, the Food and Drug Administration approved the use of lithium for the treatment of mania and the preven tion of bipolar disorder relapse7.
Lithium acts on multiple neurological circuits, causing an increase of acetylcholine concentration, synthesis and turnover in the cholinergic system and a decrease of dopamine and noradrenaline concentration, storage and release in the catecholaminergic system. It also produces an increase of tryptophan uptake at synapses levels, increasing serotonin synthesis8 and due to genomic and non-genomic events, lithium regulates electrical mem brane excitability4. All these actions are aimed to stabilize mood. In addition to its actions on the central nervous system, lithium has systemic repercussions, affecting multiple organs such as the kidney, heart, motor endplate and thyroid and parathyroid glands9.
This psychotropic drug has a narrow therapeutic range from 0.7 to 1.4 mEq/l, being toxic with plasma levels higher than 2 mEq/l and lethal with serum levels over 2.5 mEq/l10.
Therapeutic recommended doses are between 900 and 1500 mg per day11. But even within therapeutic doses, it may cause a number of adverse effects such as nausea, diarrhea, weight gain, fine tremor and skin lesions, espe cially acne and psoriasis12. It can also cause nystagmus, myasthenia gravis, papilledema, photophobia or dry eye. Other less frequent adverse effects can occur even at recommended doses such as albuminuria, hypotension, cardiac arrhythmias, QT prolongation and ST segment and T wave changes13. At toxic levels several neurologic reactions have been described such as cognitive altera tions, dysarthria, impaired coordination, mental confusion, hyperreflexia, intense tremor, focal signs, seizures, coma or even death14.
In addition to these alterations, lithium causes endo crine disorders such as thyroid disorders, hyperparathy roidism and nephrogenic diabetes insipidus that will be updated in this review. It is very important to consider these potential endocrine abnormalities when prescrib ing lithium.
Lithium and the thyroid gland
Lithium has many effects on thyroid physiology. It can cause hypothyroidism, hyperthyroidism, goiter and oph thalmopathy. These side effects are caused by different mechanisms.
Lithium accumulates in thyroid tissue by active trans port11 and the concentration can be 3 to 4 times higher than in plasma15.
It increases intrathyroidal iodine content and this could be due to thyroid-stimulating hormone (TSH) secretion as a result of lithium-induced hypothyroidism16. Furthermore, lithium may compete for iodine transport resulting in low thyroid iodine uptake, which is reversible and dose-dependent17.
Lithium also inhibits the coupling of iodotyrosine resi dues to form iodothyronines18-20 and inhibits the release of T4 and T319-21, which is the main mechanism involved in hypothyroidism and goiter11. The mechanism of hormone release inhibition involves an alteration in tubulin polymer ization as well as inhibition of the action of TSH on cyclic adenosine monophosphate production22.
This cation reduces thyroid iodine uptake, interfering with tyrosine iodination, changing thyroglobulin structure and colloid formation in the apical pole of thyroid cells, thus interfering with iodotyrosine synthesis. Lithium decreases in vitro colloid droplet formation within thyroid follicular cells, a reflection of decreased colloid pinocytosis from the follicular lumen23,24.
The significant decrease of plasma T4 clearance in patients receiving lithium may be due to inhibition of thyroid hormone secretion, thereby inducing a decrease of type I 5’ deiodinase activity (DIO1)25 (Fig. 2).

Fig. 2 Lithium actions in the thyroid gland: reduced iodine uptake, inhibitory effect on the cAMP pathway, changes in thyroglobulin structure and colloid formation in the apical pole of thyroid cells, interference in tyrosine iodination and inhibition of T4 and T3 release
It also inhibits type II deiodinase enzyme (DIO2), lead ing to a decrease in pituitary T3 concentrations26.
Goiter
Up to 50% of patients on chronic lithium treatment may develop goiter27. The prevalence of goiter is higher in long-term users and in those patients living in iodine-deficient areas. It is usually a diffuse, painless and benign goiter28. The inhibition of thyroid hormone secretion by lithium results in decreased serum T4 and T3 concentrations, with a compensatory increase in pituitary secretion of TSH, leading to thyroid enlargement and secretion of a normal amount of thyroid hormone by an enlarged thyroid gland16,20. Goiter occurs more frequently within the first two years of treatment29.
Thyroid enlargement may also be due to insulin-like growth factor alterations induced by lithium, post-receptor tyrosine kinase pathway and/or Wnt/beta-catenin signal ing16,30.
The management should be the same as in any patient with goiter and it would be a good practice to perform a thyroid ultrasound before starting lithium29.
Hypothyroidism
Up to 30% of patients chronically treated with lithium develop increased TSH that may progress to overt hypo thyroidism. It usually occurs during the first two years of lithium therapy31 and is more frequent in women over 45 years old, with an increasing risk with age32,33. It is revers ible with the discontinuation of lithium9.
If hypothyroidism develops, treatment with levothyrox ine should be started. There is no need to stop treatment with lithium, but if lithium is subsequently discontinued, it is reasonable to reassess the need for continuing thyroid hormone replacement. Some guidelines suggest measuring TSH two months after withdrawal29. If TSH is in the lower half of the normal range or below normal, levothyroxine should be discontinued and TSH and free T4 should be reassessed in six weeks. If TSH rises above normal, levothyroxine may be restarted, depending on free T4 level and clinical assessment. On the contrary, if the TSH is in the upper half of the normal range or above normal after lithium withdrawal, levothyroxine should be continued29.
There is a strong recommendation for measuring thy roid function and antibodies in all patients prior to lithium therapy regardless of gender or age34,35. Besides, it is reasonable to perform annual thyroid function tests in patients receiving lithium33.
Autoimmune thyroiditis
It is not clear if lithium itself can induce autoimmunity. It is likely that many patients who develop hypothyroidism during lithium treatment have underlying chronic autoim mune thyroiditis18,20,36.
Some studies have shown that it can accelerate the development of preexisting thyroiditis. The drug does not seem to be able to stimulate the production of thyroid antibodies de novo in humans, but there is evidence that lithium therapy is associated with a rise in antibody titer in patients who already have positive antibodies at the beginning of the treatment34.
Hyperthyroidism
Hyperthyroidism is a rare complication of lithium treat ment with an incidence range from 0.1-1.7%37. In patients treated with lithium, the frequency of hyperthyroidism was two to three times higher than in the general population38,39.
One study proposes that lithium induced thyroiditis is a consequence of maladaptation to disturbed iodine kinetics with an escape phenomenon after expansion of the intra thyroidal iodine pool38. In a review of 21 published cases of lithium induced thyroiditis, Lazarus concluded that besides an autoimmune mechanism, diffuse hyperplasia might be the main cause of this manifestation22. Furthermore, lithium could cause direct damage to thyroid cells, with the consequent release of preformed hormones into the bloodstream; a mechanism similar to that of amiodarone-induced thyroiditis40.
The etiology of hyperthyroidism includes Graves’ disease, toxic nodular goiter and silent thyroiditis16 and should be accordingly treated.
Ophthalmic adverse effects
In patients taking lithium, thyroid eye disease is most com monly seen in hyperthyroidism, but it can also be seen in hypothyroidism and euthyroid state41.
The most common signs and symptoms are eyelid retraction, exophthalmos, diplopia, ocular injection, lag ophthalmos, dry eyes and vision loss42.
Some patients develop exophthalmos even within therapeutic serum lithium range43. The exophthalmos produced by lithium does not have the retroorbital infiltra tive changes seen in Graves’ disease. Discontinuation of lithium leads to improvement of exophthalmos over the course of several months43,44.
Further research is warranted to guide clinical decision-making in managing patients presenting with exophthal mos in the setting of lithium treatment45.
Treatment with lithium in thyroid disease
Lithium has been used for many thyroid disorders, even though is not considered a first line treatment.
Hyperthyroidism: lithium can be used for the treatment of hyperthyroidism in doses between 600 to 1000 mg a day in patients allergic to iodine46,47, with intolerance or lack of response to thionamides48 and in amiodarone in duced thyrotoxicosis49.This treatment leads to a decrease in serum thyroid hormone concentrations and clinical improvement50,51.
Thyroid cancer: as mentioned previously, lithium could lengthen iodine retention after radioiodine thus increasing its effectiveness52. However, in the absence of clinical trials showing a beneficial effect, American Thyroid Associa tion (ATA) does not suggest using lithium as an adjunct to radioiodine53.
Hypercalcemia and hyperparathyroidism
The prevalence of lithium-induced hyperparathyroidism ranges from 6.3% to 50% in those patients who require long-term therapy54 and its incidence is higher in women (4:1)11. The prevalence is difficult to establish because it is undiagnosed in asymptomatic cases and because the assays employed to measure PTH or calcium vary in different studies.
According to a literature review, there have been pub lished 84 cases since 1973, between 21 and 84 years. Among these patients, 42 had adenomas and 29 had diffuse parathyroid hyperplasia55.
It has been shown that PTH increases 30%, calcium 2.5% and magnesium 5% with lithium treatment compared to their baseline values56. These increases do not seem to be related to the duration of therapy, as cases have been reported in patients treated for only 4 weeks57, nor have they been associated with the dose of lithium or derived from its toxicity58.
Pathogenesis
Lithium has direct actions on parathyroid glands antago nizing the calcium sensing receptor (CaSR): it increases the calcium threshold necessary to decrease PTH se cretion thus reducing the suppression of PTH by serum calcium. It remains to be elucidated whether the lithium ion binds directly to the CaSR or affects its function through a different mechanism. By shifting the PTH/calcium secre tion curve to the right, higher serum calcium levels are required to inhibit PTH secretion, finally increasing both their levels59.
It also has indirect actions promoting the reduction of urinary calcium excretion due to the increase in renal reabsorption secondary to PTH increase, and also causing an increase in intestinal calcium absorption60.
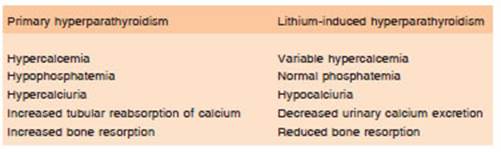
Patients who develop lithium-induced hyperparathy roidism typically have serum calcium levels ranging from slightly above the normal range to more than 15 mg/dl and PTH levels ranging from high normal to several times the upper limit of normal. In cases where the PTH level is within the normal range, it is generally higher than would be expected for a given serum calcium level because the parathyroid glands normally respond to hypercalcemia by suppressing PTH secretion below the lower limit of reference54. In lithium-associated hyperparathyroidism, renal cyclic AMP levels are low or normal, serum phos phate levels are usually in the normal range and serum magnesium levels are elevated. Unlike primary hyper parathyroidism, lithium-induced hyperparathyroidism is associated with hypocalciuria and, consequently, is less likely to present with kidney stones61. Mak et al reported a series of 53 patients who were followed for two years after the beginning of lithium therapy and all developed elevations in PTH and significantly decreased fasting and 24-hour urinary calcium excretion, suggesting a reduction of bone resorption62.
The characteristics that differentiate primary hyper parathyroidism from secondary hyperparathyroidism due to lithium treatment are shown in Table 1.
Diagnosis and treatment
Monitoring of calcium levels is recommended before start ing lithium therapy and subsequent monitoring 2-6 weeks after starting it. After this initial follow-up, annual calcium control is suggested for surveillance. If patients remain asymptomatic, biochemical control for calcium should be performed annually63.
Once confirmation of hyperparathyroidism is reached, in the absence of a lithium overdose, the clinician must then decide between three main options: cessation of lithium and consideration of alternative psychiatric medication(s), monitoring of calcium levels while remain ing on lithium and parathyroid exploration and surgical excision of abnormal parathyroid tissue64.
Given the choice to discontinue lithium, a 28-fold higher mania relapse rate was reported 3 months later. In addition to the psychiatric consequences, there are still patients who will remain hypercalcemic after cessation of lithium therapy63.
If lithium therapy should still be continued, calcium, kidney function and bone mineral density should be monitored in all patients every 6-12 months. In selected cases the use of cinacalcet (allosteric activator of CaSR) could be an alternative to be considered65. This drug decreases the activation threshold of the CaSR by extracellular calcium and promotes a subsequent decrease in PTH secretion. To date, only five patients have been treated with cinacalcet with subsequent improvement of hypercalcemia during lithium therapy. No adverse effects have been reported and the therapy was well tolerated65,66.
Surgery is indicated in all patients who cannot be cured or when lithium therapy discontinuation is not recommended. The approach is controversial because between 25% and 75% present with multiglandular pathology67,68. Unilateral parathyroidectomy is recom mended when preoperative location of the lesion has been possible and when intraoperative PTH measure ment is available. In the absence of localization, bilateral exploration with removal only of the abnormal glands or subtotal parathyroidectomy (three and one half glands) is recommended64.
Nephrogenic diabetes insipidus
The kidneys’ ability to retain water and concentrate urine is regulated by ADH, renal medulla osmolality, adequate sodium transport and aquaporins function69.
The use of lithium is the most common cause of ac quired nephrogenic diabetes insipidus (NDI) and up to 20-40% of patients can develop it70. Its clinical presentation is gradual and is characterized by polyuria, polydipsia and low urinary osmolarity.
Aquaporins are water channels that are expressed in the renal tubules and collecting ducts. The greater the activation of aquaporins, the greater the reabsorption of water in the renal collecting ducts, which reduces the vol ume of urine. Lithium inhibits the expression of aquaporin channels in the renal collecting tubule, mainly aquaporin 2. The possible mechanisms proposed are the decrease in cAMP due to lower expression of the β isoform of the enzyme glycogen synthetase kinase or less transport of aquaporins towards the apical membranes6. This in hibition of aquaporins during lithium treatment produces polyuria70-72 (Fig. 3).

Fig. 3 Lithium actions in the kidney: Under physiological conditions, water enters the collecting duct cells through the aquaporin-2 channels and then passes into the medullary interstitial fluid. Lithium crosses the apical membrane through the epithelial sodium channel and inhibits the expression of these aquaporins in the renal collecting duct. As a result, the cell becomes at least partially insensitive to the actions of aldosterone and vasopressin, causing the excretion of dilute urine (decrease osmolality)
This adverse effect is usually reversible after drug withdrawal, however some patients may present ir reversible kidney damage due to chronic interstitial nephropathy6,73.
To prevent renal toxicity, in addition to monitoring serum lithium and creatinine levels, a single daily dose of lithium should be preferred73. Thiazide diuretics are a therapeutic option in NDI; however, hydrochlorothiazide has the potential to increase lithium toxicity and should be used with caution in these cases. Amiloride would be a better option because in addition to its natriuretic action (which causes the contraction of extracellular volume, the consequent decrease in glomerular filtration and ultimately leads to a decrease in urine volume), it also reduces the entry of urine lithium in distal tubule cells6,74.
In conclusion, lithium therapy continues to be one of the most prescribed medications to treat bipolar disorders. However, the adverse effects induced by lithium over the endocrine system are quite common. Therefore, it is es sential for physicians to know how to carry out a complete and adequate clinical and biochemical analysis for the early identification of these disorders. The withdrawal of lithium or its replacement by another treatment should be evaluated in the presence of endocrine dysfunction.