KEY POINTS
• The safety and effectiveness of combination therapy with tocilizumab and corticosteroids in severe SARS-CoV-2 pneumonia is known.
• In this cohort tocilizumab showed an adequate safety profile.
• Its efficacy in decreasing inflammation parameters has been demonstrated, although decreased in mortality doesn’t seem to be as effective.
SARS-CoV-2 infection has a wide variety of clinical presentations as well as systemic involvement; respiratory failure being the most severe one. Respiratory distress syndrome is the main cause of mortality, and massive release of pro-inflammatory cytokines may contribute to poor prognosis1.
In Argentina, since March 2021, an increase in coro navirus infection, which continued until end of August, has been observed, with peaks of approximately 40 000 daily cases by end of May2. This increase in COVID cases resulted in a larger number of severe cases and deaths, as well as a high occupancy rate at Intensive Care Units.
Until that moment, standard of care (SOC) for patients with severe or critical infection (WHO Severity Ordinal Scale, category 6-7)3 included oxygen support (non-rebreather mask, high flow nasal oxygen [HFNO] or inva sive ventilation [IMV]), thromboembolic prophylaxis and 6 mg of systemic dexamethasone (oral or intravenous)4,5.
The RECOVERY6 and REMAP-CAP 57 studies demon strated that using tocilizumab (TCZ) in combination with systemic corticosteroids decreased mortality in people with SARS-CoV-2 pneumonia with high oxygen requirements.
Based on these publications, United States’ National Institutes of Health (NIH)8 and Infectious Diseases Society of America (IDSA)9 made recommendations for its use.
In May 2021, Argentina’s Health’s Ministry published a TCS assessment report, which concluded that its use may potentially decrease mortality and need for invasive mechanical ventilation (IMV) in patients with severe COVID-1910.
This study intended to investigate about the clinical and laboratory features, potential impact on mortality, occurrence of secondary infections and clinical outcome as per WHO Ordinal Scale at discharge (or at 14 days, whichever occurs first).
Materials and methods
Between March and August 2021, an observational and ana lytical study of a retrospective cohort of adult patients with severe or critical SARS-CoV2 pneumonia (WHO Ordinal Scale3 categories 6 and 7) hospitalized at Sanatorio Finochietto was conducted. Patients were classified according to whether they received standard of care (SOC) treatment (dexamethasone 8 mg or equivalent plus oxygen therapy, high flow nasal oxygen [HFNO] or IMV) or SOC plus TCZ (8 mg/kg, single dose).
TCZ was administered based on the following criteria:
- SARS-CoV2 pneumonia confirmed by PCR.
- requirement of being hospitalized and requiring oxygen by non-rebreather mask, high flow nasal oxygen or invasive mechanical ventilation.
- corticosteroid treatment for at least 24 hours prior to clinical worsenig (dexamethasone, at least 8 mg daily or equivalent).
- rapidly progressive clinical worsening (requiring more than 5 L/min of high flow nasal oxygen or non-rebreather mask).
- plus any of the following factors: increasing CRP or a total value of more than 75 mg/dL, 60 years or older, diabetes, chronic obstructive pulmonary disease, high blood pressure, obesity.
The following were considered contraindications for use:
- neutropenia (neutrophils less than 1000/mm3),
- liver enzymes (ALT/TGP: glutamic pyruvic transaminase and AST/TGO: glutamic-oxaloacetic transaminase) at least five times the normal value,
- severe immunosuppression (oncological disease receiv ing chemotherapy treatment, patients with solid organ or bone marrow transplant) and/or
- suspected or confirmed secondary infection (bacterial or fungal) at time of clinical worsening.
Sanatorio Finochietto, in Buenos Aires City, Argentina, is a Level 3 health center that provides health care services for prevalent clinical and surgical conditions present. This institution has an Emergency Department, Maternity Ward, General Ward and Intensive Care Unit, with a total of 180 beds; electronic clinical records and high-complexity complementary services.
Treatment protocol was approved by Sanatorio Finochi etto’s Ethics Committee under Number 5489 and registered in ClinicalTrials.gov (No. NCT05057962).
Statistical analysis
In the descriptive analysis, quantitative data is expressed as median and interquartile range (IQR) 25-75. Qualitative data is expressed as absolute frequency and relative percentage. Chi2 method or Fisher test were used to compare groups. For comparison between measures in each treatment group Wilcoxon signed rank test was used.
Multiple organ failure was analyzed using logistic re gression. Multiple logistic regression incorporating all significant baseline variables was used to derive a pro pensity score or the predicted probability that an individual patient belonged to each treatment group. The propensity score included: age, obesity, arterial hypertension, CRP worsening and HFNO. Comparisons between groups re garding outcome variables was then performed adjusted for significant baseline variables and propensity score in multivariable regression analysis. The percent variation in the WHO Ordinal Scale was analyzed using lineal regres sion. Significant level was set less of 5% and software R was used for analysis.
This study was conducted following national and interna tional regulatory standards on research on human subjects; and following Health Ministry Resolutions, Helsinki Decla ration including all its amendments and Clinical Practice Guidelines (ICH E6). All study data was collected from electronic clinical records and was managed following high confidentiality standards, and anonymously, with restricted access and only for study purposes as per the current leg islation on National Personal Data Protection Laws (Habeas Data Law) and Law 26529/09 of Argentine Republic. The study was approved by Sanatorio Finochietto’s Independent Ethics Committee.
Results
During the analyzed period, 101 patients with severe or critical SARS-CoV-2 pneumonia were hospitalized. Of these patients, 45.5% (n = 46) (Ci95% 35.6-55.7) received SOC and 54.5% (n = 55) (Ci95% 44.2-64.4) SOC plus TCZ. Of the latter, 92.7% (n = 51) were analyzed and 7.3% (n = 4) were excluded due to miss ing data as they were transferred to other health care centers. On admission, patients had median values of 6.00 [6.0-7.0] on WHO Ordinal Scale, 119.0 mg/dL [65.0-171.0] on CRP, 1661.0 ng/mL [931.0-2644.0] on ferritin and 0.30 ug/mL [0.23-0.45] on D-dimer. High flow nasal oxygen (HFNO) was required by 59.8% (58) (Ci95% 49.3-69.2) of patients as only treatment or prior to invasive mechanical ventilation, which was necessary in 55.7% (54) (Ci95% 45.2-65.7) of the cases. Patients received systemic dexamethasone 8 mg for a median of 13.0 days [10.0-17.0].
Shock, requiring vasoactive drugs for more than 24 hours, was present in 28.1% (27) (Ci95% 19.2-37.8) of patients, and 18% (18) (Ci95% 11.3-27.7) had multiple organ failure.
Survival rate was 81.4% (83) (Ci95% 76.9-91.8); with a median hospital stay of 19.00 days [13.0-28.0].
There were similar features between both groups (asthma, COPD, diabetes, arterial hypertension, coronary artery disease, heart failure, cancer, HIV) and some dif ferences (age and obesity). Severity at time of admission, APACHE II score and WHO Ordinal Scale were similar in both groups, as well as ferritin and D-dimer values. CRP values were different in both groups, although this differ ence could be related to criteria for TCZ indication (CRP >75 mg/dL). All data is shown in Table 1.
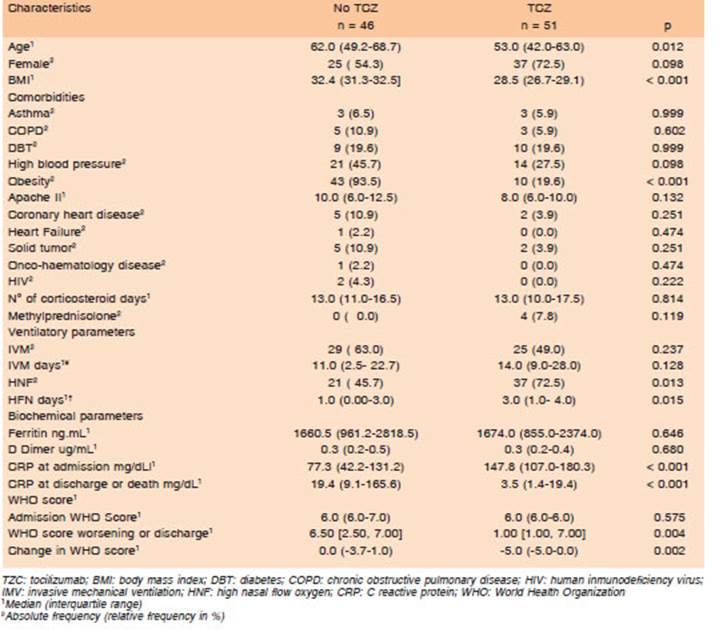
Table 1 Comparison of demographic, comorbidities, ventilatory, biochemical parameters and outcomes in patients based on whether they received tocilizumab treatment or not
TCZ was administered in 52.6% (51) (Ci95% 42.2-62.8) of cases as a single 800 mg [600.0-800.0] dose within 24 hours of clinical worsening, and with an average time of administration of 10.0 days [9.0, 11.5] from symptom onset. None of these had to be discontinued due to acute adverse effects during infusion. The 15.6% (Ci 95% 7.0- 28.5) 8/51 of patients had side effects in the following days (48 hours or 7 days, increased liver enzymes at least five times the normal value with no liver failure). Neutropenia was not observed in any case.
Patients in the TZC group had a lower CRP value at discharge with a higher delta from time of worsening to discharge (Fig. 1), lower rate of multiple organ failure, better functional status at discharge and shorter hospi talization period.
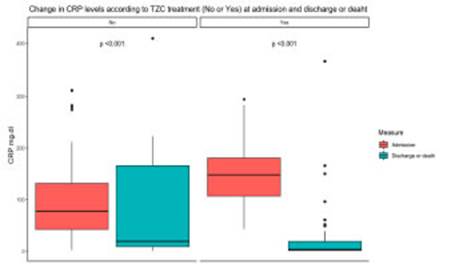
Fig. 1 Change in C Reactive Protein level based on tocilizumab treatment (No or Yes) on admission and at discharge or death
Bivariate analysis of primary objective showed no dif ferences in mortality rate and secondary infection occur rence. When assessing clinical status as per WHO Ordinal Scale there was a significant difference in its variability from worsening to discharge (or 14 days), evidencing better functional status in patients receiving TCZ (Fig. 2).
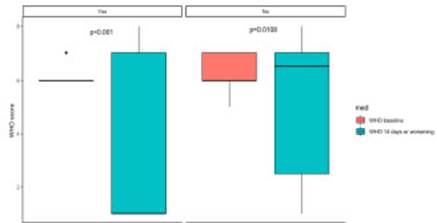
Fig. 2 Change in WHO Ordinal Scale based on tocilizumab treatment on admission and at discharge or death
Regarding secondary objectives, there were no differ ence on mechanical ventilation days or shock occurrence. Data about primary and secondary objectives are shown in Table 2.

Table 2 Comparison of outcomes in patients based on whether they received tocilizumab treatment or not
Multivariate analysis showed no differences in multiple organ failure and TZC treatment after adjustment by HFNO and propensity score (odds ratio 2.81 Ci 95% 0.62-14.62 p = 0.196). There were no differences in WHO Ordinal Scale score variation after adjusted for obesity, high blood pleasure, CRP on admission and HFNO (beta -1.20 Ci95% -2.76-0.23 p = 0.096) neither.
About half of the patients (47.4%-n: 46) presented secondary infections, but no significant differences were found between both groups (SOC 50.0% (23) vs. SOC + TZC 45.1% (23) (Ci95%31.1-59.6, p: 0.78) (Fig. 3).

Fig. 3 Secondary Infections (except ventilator associated pneumonia). Comparison between patients based on whether they received tocilizumab treatment or not
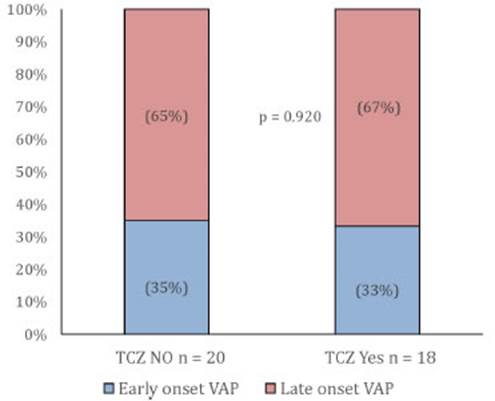
When classifying infections by type of event and com paring them between groups, there were no differences in early-and late-onset ventilator-associated pneumonia (Fig. 4), but there was higher incidence of urinary tract infections and catheter-related infections in SOC group, and of primary bacteremia in SOC + TCZ group.
Discussion
This study is the continuation of our recent report on our initial experience using TZC in 30 patients hospitalized due to severe or critical SARS-CoV-2 pneumonia11.
Regardless of the treatment administered, in this co hort we observed a lower mortality compared with prior publications in Argentina12. This could be related to better knowledge and experience gained in the management of these patients. Locally, this might also be related to the fact that patients involved in the second wave in our country were younger, but it does not seem to be related to tocilizumab use.
Patients that received TZC in the analyzed period did not present any of the most frequently reported adverse effects (reaction during administration, neutropenia or liver toxicity); confirming its safety profile.
In hospitalized patients with severe COVID, variable rates (3.7 up to 43%) of secondary infections have been reported. The impact of using immunosuppressive or immunomodulatory drugs in their occurrence is under discussion. Several controlled trials demonstrated that the number of infections was not higher with its use13. Secondary infections in our cohort are closer to the upper levels of these values.
A recently published multicenter retrospective cohort report14, described a higher probability of bac teremia when using the combination of corticosteroids plus tocilizumab [OR 2.13, 95% CI 1.2-4.05, p-value 0.0217]. In a similar way, our combination therapy group (dexamethasone plus TCZ) presented a higher incidence of primary bacteremia. This doesn’t seem to be a prognostic factor since it was not associated with an increase in multiple organ failure, shock or mortality. Regarding other kinds of infections, ventilator-associ ated pneumonia rate was similar in both groups; and we did not find an association between tocilizumab and developing fungal, viral or other multidrug-resistant germ infections.
Secondary infections do not seem to be a worse prog nostic factor; mortality was not affected by the presence of secondary infections and remained relatively low when compared to averages in our country, and in international reports15.
CRP values were significantly higher in the group receiving TCZ. This was expected since having a CRP value over 75 mg/dL was an eligibility criterion. There is a clear evidence of Its activity as moderator of the hyper inflammatory response since, in spite of having initial higher levels, the values of this biomarker at time of dis charge were significantly lower in the combination therapy group, with a delta between time of clinical worsening and discharge, favoring the latter.
Regarding severity, both groups were similar as its shown in Apache II and WHO Ordinal Scale values at time of worsening. As previously mentioned, TCZ group had a lower average age and BMI.
In a bivariate analysis, we found a difference in development of multiple organ failure and condition at discharge, favoring combination therapy group, with an im portant improvement in WHO Ordinal Scale score. When performing a multimodal analysis with these mentioned variables, this apparent protective effect disappears, re flecting what is well described in the literature about age and obesity being poor prognostic factors for patients with severe SARS-CoV-2 pneumonia16-20.
In May and June, due to sustained high number of cases, prolonged hospitalizations and limited resources, Intensive Care Unit occupancy rate was 90% at national level21. In this context, number of hospital stay days were also a factor to be considered. In combination therapy group we observed an average of 2.5 less days of hos pitalization.
This study has limitations, the most important ones, being those related to the retrospective and observational cohort design and that the number of patients included in the analysis, may be little to draw solid conclusions. However, we believe it adds information on the use of tocilizumab and raises the question on whether its use is equally effective in our setting to that reported in other case series. In order to answer this question, it would have been interesting to propose a prospective cohort, which was not possible in that epidemiological setting.
In conclusion, the use of tocilizumab in combination with systemic corticosteroids is considered a safe and efficacious therapy to treat severe or critical SARS-CoV-2 pneumonia.
In our cohort, adding a single dose (8 mg/kg) of tocili zumab to systemic corticosteroids in patients with severe or critical SARS-CoV-2 pneumonia seems to maintain that described safety profile. However, we were able to demonstrate its efficacy only in decreasing inflammation biomarkers and a tendency to fewer hospital stay days; with no impact on mortality or clinical outcome. This raises the question of the cost-benefit of its use in our setting.