KEY POINTS
Current knowledge
• In multiple sclerosis, most approved vaccines induce robust humoral and cellular immune responses against the SARS-CoV-2.
• However, it is still unknown whether all anti-SARS-CoV-2 vaccines commonly used in Latin America (Sputnik V or AstraZeneca for example) could elicit serological response in multiple sclerosis patients treated with disease-modifying therapies
Contribution of the article to current knowledge
• We provide evidence that multiple sclerosis patients receiving disease modifying treatment that received Sputnik V or AstraZeneca vaccines for SARS-CoV-2 developed a serological response with no differences between the vaccines used.
• Our study contributes to understanding the immunological response to COVID-19 vaccines in multiple sclerosis patients receiving disease modifying drugs where the drug response could be affected.
Different vaccines have been evaluated and used to achieve immunization against COVID-19 in the world1-4.
In multiple sclerosis (MS), most approved vaccines induce robust humoral and cellular immune responses against the SARS-CoV-2 virus spike protein1,5-7. However, it is still unknown whether all anti-SARS-CoV-2 vaccines commonly used in Latin America like Sputnik V (Gam-COVID-Vac-rAd26/rAd5) and AstraZen eca (AZD1222/ChAdOx1) could elicit serological response in MS patients treated with disease-modifying therapies3.
RelevarEM is a nationwide, multicenter regis try of patients with MS in Argentina8,9. It collects data from daily clinical practice in this popula tion. This approved registry monitors the func tional health status of almost 4000 patients in the country8,9.
The objective of our study was to assess the immunogenicity, effectiveness, and safety against SARS-CoV-2 vaccines in a subset of MS patients included in RelevarEM.
Materials and methods
The present investigation was a prospective cohort study that started in May 2021 and finished in December 2021. The study was run on the current MS Argentinean registry, RelevarEM8,9. RelevarEM is a longitudinal, strictly observational multiple sclerosis (MS) and neuromyelitis optica spectrum disorders (NMOSD) registry in Argentina. It is open to all practicing neurologists and MS specialists and their teams across the country. The registry tracks the outcomes of routine clinical practice for MS and NMOSD patients in a web-based platform that allows researchers to register and follow up individuals. The primary objec tive of the registry was to create an MS physician net work in Argentina that captures pragmatic and relevant information from MS patients in terms of clinical and demographic aspects8,9. Eligible subjects were contacted by their neurologist. Upon patient recruitment, we col lected data about demographic and clinical characteris tics of participants (age at vaccination, Expanded Disabil ity Status Scale (EDSS) at study entry, ongoing treatment, MS phenotype, and comorbidities); vaccine received as a first and as a second dose, dosing, and intervals; adverse events of vaccination and follow-up time. All patients were actively followed for at least three months since the second COVID-19 vaccine dose (suggested immunization schedule completed).
The primary outcome of the study was to measure the immunogenicity of anti-SARS-CoV-2 vaccines. Second ary outcomes included measuring the immunogenicity of anti-SARS-CoV-2 vaccines stratified by vaccines used and ongoing MS treatments and finally to measure the effectiveness (COVID-19 cases in post-vaccination MS pa tients) of vaccines in all the MS patients included during 3 months after their vaccination. Clinical and demographic data were obtained from treating neurologists. Informa tion was collected by communication between physician and patient to find out whether infection had occurred in the 3-month follow-up period. If that was the case, spe cific information regarding the infection was requested from the treating physician (date, symptoms, need for hospitalization, assisted ventilation, treatment, and evo lution). Physician contact was made proactively every 30 days during the 3-month period after vaccine schedule completion10. Immunogenicity was evaluated based on the detection of total antibodies (Ab) against spike pro tein and neutralizing Ab in serum11. The moment of de tection was 4 weeks after the second dose of the vaccine, and this process was applied both for viral vector-based vaccines (Sputnik V, AstraZeneca, CanSino and Janssen), mRNA-based vaccines (Moderna and Pfizer), inactivated vaccines (SinoVac, SinoPharm and Bharat) and vaccines based on recombinant protein nanoparticles (Novavax). All patients included in the study underwent a laboratory test 4 weeks after the second vaccination for quantita tive and qualitative detection of neutralizing antibod ies, anti-spike antigen antibodies, and total antibodies. The test was conducted at the Instituto de Investigaciones Biomédicas en Retrovirus y SIDA, UBA-CONICET (INBIRS) of the University of Buenos Aires Faculty of Medicine, a laboratory accredited in Argentina to perform the test12. A positive COVID-19 case was defined according to the criteria established by the Ministry of Health in Argentina: a detectable SARS-CoV-2 specific response by PCR or positive antigen test or a close contact with a COVID-19 case and compatible symptoms. Safety data for the first three months after the first dose of the vac cine, adverse events related to vaccination (local and/ or systemic reactions), as well as clinical activity of the disease (relapses) occurring in the first three months af ter the first vaccination were evaluated. These data were collected by communication between treating physician and patient. The contact was made proactively every 30 days during the 3-month period after vaccine schedule completion.
As for the main objective of the study (immunogenic ity), total IgG antibodies against spike protein and RBD were tested 4 weeks after the second dose in fully vac cinated individuals (STEP 1). Qualitative (positive or nega tive) results were obtained (Fig. 1). Total IgG antibodies were qualitatively measured by COVIDAR ELISA (Labo ratorio Lemos, Argentina). If antibodies were detected in STEP 1, neutralizing antibody titer was determined as de scribed below (STEP 2).
Determination of neutralizing antibodies (STEP 2) was performed by ELISA in samples with detectable anti- SARS-CoV-2 antibodies (positive STEP 1). Briefly, serial two-fold dilutions were incubated with 200 SARS-CoV-2 plaque-forming units (PFU) for 1 hour at room tempera ture, in triplicates. The mixture was then added to 80% confluent Vero-E6 cell monolayers in 96-well plates and incubated at 37 °C for 1 hour. Then, cells were washed and culture medium containing 2% FBS was added. After 72 hours, plates were fixed with 4% paraformaldehyde for 20 minutes at room temperature and stained using a 0.5% crystal violet dye solution in acetone and methanol. The neutralization titer was calculated as the reciprocal of the highest plasma dilution that showed 80% inhibition of the cytopathic effect.
A specific form was developed to collect data for the research project. The form was added to the RelevarEM registry as a vaccination supplementary form to follow every patient.
Analysis was conducted for both vaccination sched ules and treatment strategies used in the patients includ ed in the study.
Sample
All MS-diagnosed patients included in RelevarEM who received any approved SARS-CoV-2 vaccine were invited to participate in the study. To reduce the probability of selection bias, we sought to include all professionals in Argentina who oversaw healthcare of MS patients in Ar gentina and were active members of RelevarEM.
Each center submitted the project for approval follow ing the local regulations in force.
An institutional ethics committee approved the center registration or declared its exemption of approval, as well as the informed consent (IC).
Statistical analysis
Baseline characteristics of the cohort were reported in percentages for categorical data and in median and range or mean ± SD standard deviation for continuous data. Only participants with a complete vaccination schedule (at least two doses) and a minimum three-month follow-up period were included in the analysis. In order to com pare categorical variables, chi-squared test or Fisher’s exact test were used. T-test was performed for continu ous variables with normal distribution, and Wilcoxon or Kruskal-Wallis tests were used for variables of non-nor mal distribution, according to the variance homogeneity between groups. Linear correlation between continuous variables was calculated by the Spearman method. All tests were two-sided, with a 0.05 alpha risk. Statistical analysis was performed using R Studio, version 3.5.2 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 94 patients were included during the study period. The mean age at study entry was 41.7 ± 12.1 years. Most individuals were relapsing-remitting MS patients (RRMS) (n = 80, 85.1%). The majority were female (n = 63, 67%) and the median Expanded Disability Status Scale (EDSS) was 2 (SD 1.3, range 1-6.5). The most frequently used treatment was fingolimod, ob served in 30 (31.9%) patients. Patients had a low comorbidity score (Charlson score index 0.08, SD 0.32). Ten patients (10.6%) were infected with CO VID-19 prior to study inclusion from May 2020 to June 2021. In those patients, infection was confirmed by PCR testing. Among previously infected patients, only 1 (0.01%) was hospital ized (Table 1). All patients included were fully vaccinated. In 33 patients (35.1%), Sputnik V vaccine was used as the first dose, while 61 patients (64.9%) received AstraZeneca in the same instance (Table 1). As for the second dose received, in 32 patients (34%), the vac cine administered was Sputnik V and 62 (66%) were given AstraZeneca (Table 1). No other vaccines were administered to the sample of patients despite other vaccines were avail able in Argentina at the time of the study. We analyzed patients with homologous (Sputnik V-Sputnik V or AstraZeneca-AstraZeneca) or heterologous (Sputnik V-AstraZeneca or As traZeneca-Sputnik V) vaccination schedules. Ninety-two-point five percent of patients completed a homologous schedule (Table 1). Clinical and demographics variables are shown in Table 1.
Serological response
We analyzed the humoral immunogenicity in terms of presence of antibodies (STEP 1) and functionality (STEP 2) as described in the statis tical plan. Detection of total antibodies against
RBD and spike protein was performed in all in dividuals. We found that 60 participants (63.8%) were able to develop a specific humoral re sponse after vaccination, while a negative result was obtained in 34 patients (36.2%) after two vaccine doses (Table 2). In all participants with a positive response in STEP 1, neutralizing an tibodies were quantified (STEP 2). We stratified immunological response according to the vac cination schedules that individuals received, as follows: Sputnik V or AstraZeneca homologous schedules (both doses of the same vaccines), and heterologous schedules. We observed no differences in qualitative response between vac cines or combination received (Sputnik V and AstraZeneca, p = 0.87, (Table 2). We also stratified the analysis for MS treatment groups. Despite the low number of patients in each group, a significantly low number of subjects developed antibodies against spike antigen in the group of patients treated with ocrelizumab, and a lower number of subjects developed antibodies in the group receiving fingolimod compared to other group of treatments (Table 3).

Table 3 Proportions of patients with serological response (antibodies against spike antigen) according to vaccines and disease-modifying therapies
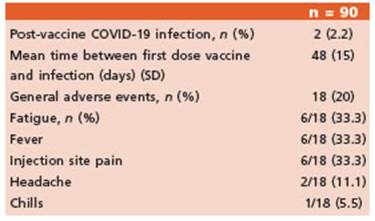
During the three-month follow-up, two indi viduals were diagnosed with COVID-19 by PCR testing. One patient was receiving fingolimod and the other was on ocrelizumab treatment. None of them required hospitalization or oxy gen supply.
No MS relapses during the three-month fol low-up were reported in patients after the com plete vaccination schedule.
Safety data were obtained from 90 patients. A total of 18 (20%) reported adverse events after vaccination. Most common events were fatigue (33.3%), fever (33.3%) and injection site reaction (33.3%). There were no significant differences in the frequency of adverse events between both vaccines (Table 4).
Discussion
This is the first study in Argentina and one of the few in the Latin American region to evaluate the immunogenicity, effectiveness, and safety against SARS-CoV-2 vaccines in a subset of pa tients with MS.
Several findings must be highlighted. Much information about Pfizer and Moderna vaccines has been gathered1,5-7,10,11, and this study pro vides a comprehensive overview of COVID-19 vaccine use in MS patients in the Latin America region. Individuals with heterologous vaccina tion schedule were included in the study. This strategy was implemented in Argentina to solve a problem of vaccine availability and the limited data about safety, efficacy, and immunogenic ity of COVID-19 vaccines. It is important to note that, despite the low number of patients includ ed, there are more cases under investigation. A considerable number of patients (almost 64%) developed total antibodies (Ab) against spike protein after COVID-19 vaccination. It should be mentioned that, due to availability of vaccines in Argentina between June and September 2021, a heterologous scheme of vaccination (the possibility to combine doses of vaccines, i.e., Sput nik V and AstraZeneca and Sputnik V and Mod erna or Pfizer) was available. This allowed us to analyze a small number of patients. However, the overall immunological response was lower than that observed in clinical trials of both vac cines in general population (98.2% and 99% for Sputnik V and AstraZeneca, respectively)13,14. In Argentina, no previous studies were carried out in MS patients to evaluate vaccine response, but some data exist regarding the response to Sput nik V and AstraZeneca in general population. A study evaluated SARS-CoV-2-specific antibody responses after Sputnik V vaccination in health care workers in Argentina, measuring IgG anti-spike titers and neutralizing capacity after one and two vaccine doses in a cohort of naive or previously infected volunteers12. Around 21 days after receiving the first dose of the vaccine, 94% of naive participants developed spike-specific IgG antibodies. A single Sputnik V dose elicited higher antibody levels and virus-neutralizing capacity in previously infected individuals than in naive pa tients who received the full two-dose schedule12. As observed in previous studies, patients receiv ing anti-CD20 therapy (ocrelizumab and ritux imab) had the lowest qualitative and quantitative response against COVID-19 vaccine5,10, while a reduced number of patients under ocrelizumab where evaluated in our study (n = 7).
We only observed two infections during the follow-up period and those infections were not severe. No relapses were described during the three-month follow-up after the complete vac cination schedule.
Our study has many limitations that should be mentioned. Probably, the most relevant limi tation is the modest number of patients includ ed and the observational design implemented. However, many considerations were done to avoid information bias, for instance, the way of analyzing the serological response and the strict clinical follow-up. Another limitation of the study is the absence of analysis on the cellular response to vaccines as well as the exclusion of other vaccines used in Argentina (Moderna and Pfizer). However, one of the objectives was to assess the response to vaccines in MS patients that had not been evaluated before.
In conclusion, we found that MS patients that received Sputnik V or AstraZeneca vaccines for SARS-CoV-2 developed a serological response, with no differences between the vaccines used. Only two cases of COVID-19 were identified dur ing the 3-month follow-up.
Our study contributes to understanding the immunological response to COVID-19 vaccines in MS patients receiving disease modifying drugs (DMDs) where the drug response could be affected1-4,10-13. Despite increasing evidence is being collected and the evidence-practice gap is narrowing, much remains to be done to elucidate the immune response to other vaccines and oth er factors like the ones considered in our study.