Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
El hornero
versión impresa ISSN 0073-3407
Hornero vol.24 no.1 Ciudad Autónoma de Buenos Aires ago. 2009
ARTÍCULOS
Hidden dichromatism in the Burrowing Parrot (Cyanoliseus patagonus) as revealed by spectrometric colour analysis
Juan F. Masello 1,3, Thomas Lubjuhn 2 and Petra Quillfeldt 1
1 Max Planck Institute for Ornithology, Vogelwarte Radolfzell. Schlossallee 2, D-78315 Radolfzell, Germany.
2 Institut für Evolutionsbiologie und Ökologie, Rheinische Friedrich-Wilhelms-Universität Bonn. Bonn, Germany.
3 masello@orn.mpg.de
Abstract
Bird colour perception differs fundamentally from that of humans. Birds have more cone types in the retina, including UV or violet cones, which enable them to perceive a wider spectral range. Thus, human colour perception can be deceiving when assessing functional aspects of bird plumage coloration, such as the intensity of sexual selection. In this study we measured reflectance spectra of different plumage regions of male and female Burrowing Parrot (Cyanoliseus patagonus) individuals. Although not obvious to human eyes, spectrometry revealed that adults are sexually dichromatic. Plumage regions with structure-based (blue) and structure-psittacofulvin pigment-based (green) coloration differed in achromatic brightness. In contrast, the psittacofulvin-based (red) region differed in spectral shape between the sexes. Thus, Burrowing Parrot is among the growing number of bird species which were formerly classed as sexually monochromatic based on human vision, but which are actually sexually dichromatic.
Key words: Burrowing Parrot ; Patagonia ; Psittacofulvin-based coloration ; Psittacofulvins ; Sex differences ; Sexual dichromatism ; Structural coloration.
Resumen
Dicromatismo sexual oculto en el Loro Barranquero (Cyanoliseus patagonus) revelado por medio de análisis espectrométricos
La percepción del color por parte de las aves difiere esencialmente de la de los humanos. Las aves tienen más conos en su retina, incluyendo conos sensibles al ultravioleta y violeta, los que les permite percibir un rango espectral más amplio. Por lo tanto, la percepción humana de los colores puede engañar a la hora de evaluar aspectos funcionales del color del plumaje de las aves, tales como la intensidad de la selección sexual. En este trabajo se midieron los espectros de reflectancia de diferentes regiones del plumaje de machos y hembras de Loro Barranquero (Cyanoliseus patagonus). Si bien no resulta obvio a los ojos humanos, las mediciones espectrométricas revelaron que los adultos son sexualmente dicromáticos. Regiones del plumaje con coloración basada en la estructura (azul) o con color basado en una mezcla de estructura y psittacofulvinas (verde) difirieron en el brillo acromático. En cambio, la coloración basada en psittacofulvinas (rojo) difirió en la forma del espectro entre los sexos. Por lo tanto, el Loro Barranquero es una más de un creciente número de aves que, de acuerdo a la visión humana, fueron previamente clasificadas como sexualmente monocromáticas pero que, en realidad, son sexualmente dicromáticas.
Palabras clave: Coloración basada en psittacofulvinas ; Coloración estructural ; Dicromatismo sexual ; Diferencias sexuales ; Loro Barranquero ; Patagonia ; Psittacofulvinas.
Received 20 March 2009, accepted 16 August 2009
Sexual dichromatism, defined as differences in the coloration of males and females of the same species, is thought to have evolved in response to selection pressures that differ between the sexes (Badyaev and Hill 2003; see also Heinsohn et al. 2005). Based on human visual perception, birds have traditionally been classified as sexually monochromatic or dichromatic species. However, this classification could be subjective if assessed from a human vision perspective. This is due to the fact that bird colour perception differs fundamentally from that of human beings: they have a wider spectral range and more cone types in the retina (Hill and McGraw 2006). The possession of UV or violet cones allows birds to see colours that humans cannot experience (Hill and McGraw 2006). Recent discoveries based on feather spectrometry showed that presumed sexually monochromatic birds were actually sexually dichromatic (e.g., Andersson et al. 1998, Cuthill et al. 1999, Mahler and Kempenaers 2002, Eaton 2005, Tubaro et al. 2005, Santos et al. 2006). Sexual dichromatism has often been used as a measure of the intensity of sexual selection (e.g., Møller and Birkhead 1994, Owens and Hartley 1998; but see also Badyaev and Hill 2003). In many polygamous birds, the more competitive sex develops more ornate plumage than the choosy sex, whereas large differences between the sexes in parental care lead to the development of more cryptic plumage in the caring sex. Monogamous species are usually less dichromatic (e.g., Owens and Hartley 1998).
In many Psittaciformes, the genders are indistinguishable to the human eye and hence these species have been traditionally classified as monochromatic. However, although most species appear to be monogamous (e.g., Masello et al. 2002), spectrometric measurements of plumage coloration suggests that dichromatism may be more frequent than previously thought when the UV part of the spectrum is considered (Santos et al. 2006). Psittaciformes, like several other orders of birds, possess ultraviolet-sensitive cones (UVS; Hill and McGraw 2006). Related to this, they also show ultraviolet reflectance in their plumage coloration (Burkhardt 1989, Pearn et al. 2003, McGraw and Nogare 2005, Santos et al. 2006, Masello et al. 2008) that at least one species uses in mate choice decisions (Pearn et al. 2001).
Psittaciformes have evolved a unique way to produce their bright plumage coloration. Parrots and cockatoos use lipochromes (Krukenberg 1882, Völker 1936, 1937, 1942) to produce the bright red-to-yellow hues in their feathers. These polyenal lipochromes, called psittacofulvins, have been known to science for a long time (e.g., Krukenberg 1882). Nevertheless, their biochemical identity, structure and distribution have been investigated only recently (Veronelli et al. 1995, Stradi et al. 2001, Morelli et al. 2003). In a study across 27 genera, McGraw and Nogare (2005) found that the 5 identified psittacofulvins occurred in all parrots they investigated. The absence of carotenoids reported for the plumage of Psittaciformes suggests that they avoid depositing or are unable to deposit such pigments in feathers (Völker 1936, 1937, 1942, Stradi et al. 2001, McGraw and Nogare 2004, 2005). Psittaciformes also exhibit striking blue structural colours produced by feather nanostructures and green colours, which are a combination of structural colour and yellow psittacofulvin pigments in particular arrays (e.g., Dyck 1971a, 1971b, 1992, Finger et al. 1992, Prum et al. 1999, Prum and Torres 2003). Recent studies highlighted the importance of UV-blue structural colours in sexual signalling in birds (e.g., Doucet and Montgomerie 2003, Shawkey and Hill 2005).
The Burrowing Parrot (Cyanoliseus patagonus) is a colonial Psittaciformes that, in Argentina, mainly inhabits the Monte Phytogeographical Province, a scrubland characterized by bushy steppes and xerophytes (Cabrera 1971). This species needs sandstone, limestone or earth cliffs or "barrancas" (gorges or ravines) to excavate nest burrows (e.g., Masello et al. 2001, 2006b). Recently, various aspects of the breeding biology of the Burrowing Parrot have been studied extensively (e.g., Masello and Quillfeldt 2002, 2003, 2004a, 2004b, 2008, Masello et al. 2002, 2004, 2006a, 2006b, 2008, 2009). The species has a socially and genetically monogamous breeding system with intensive biparental care (Masello and Quillfeldt 2002, 2003, 2004a, Masello et al. 2002, 2004, 2006b). The Burrowing Parrot shows slight size dimorphism, males being larger (about 5%) than females (Masello and Quillfeldt 2003). Like other parrots, they do not deposit carotenoids in their feathers (Masello et al. 2008); instead they have a psittacofulvin-based red patch in the centre of the abdominal region (Masello and Quillfeldt 2003, 2004b, Masello et al. 2004, 2008). Adult males have larger abdominal red patches than females (Masello and Quillfeldt 2003).
Recent work questioned sexual monochromatism in the Burrowing Parrot. Using a method based on human colour perception (RGB: red, green, blue; Masello et al. 2004), differences in hue of the red abdominal patch between male and female parrots were detected (Masello et al. 2004). The same study also found that the red patch is a good predictor of female body condition and male size, suggesting that the red coloration acts as a signal of individual condition, quality and parental investment (see also Masello and Quillfeldt 2004b). However, those previous studies used methods based on the human-visible spectrum (400-700 nm), while birds have a wider spectrum (320-700 nm), and thus will perceive colours differently to humans (Hill and McGraw 2006). More recently, the condition-dependence of the red psittacofulvin and blue structural colours in the Burrowing Parrot have been investigated with the use of reflectance spectrometry (Masello et al. 2008). Results suggested that the intensity of both the structural and the psittacofulvin-based colours of nestling parrots (Masello et al. 2008) was influenced by the conditions experienced during feather growth. In the present study, with the use of reflectance spectrometry, we measure red (psittacofulvins-based), green (mixed) and blue (structural-based) plumage coloration in a population of wild Burrowing Parrot of north-eastern Patagonia (Argentina). We test whether the variability observed in coloration is related to differences between the genders.
Methods
Bird sampling
The study was carried out during four breeding seasons (Oct 1998-Feb 1999, Nov 1999-Jan 2000, Nov 2001-Jan 2002, Nov 2003-Jan 2004) at the largest colony of the Burrowing Parrot located in a cliff facing the Atlantic Ocean in north-eastern Patagonia, Argentina (Masello et al. 2006b). The habitat in the surroundings of the colony belongs to the north-eastern Patagonian region of the Monte Phytogeographical Province, characterized by bushy steppes and xerophytes (Cabrera 1971).
According to accessibility, 79 nests were closely monitored in one region of the colony (41°03'S, 62°48'W) as part of an ongoing study of the breeding behaviour of the species (e.g., Lubjuhn et al. 2002, Mey et al. 2002, Blank et al. 2007, Masello et al. 2009 and references therein). Nests were inspected every five days by climbing the cliff face. Adult parrots were captured in their nests only during the breeding season and while attending nestlings.
Birds were sexed using PCR amplification of a highly conserved W-linked gene as previously described (Lubjuhn and Sauer 1999, Masello and Quillfeldt 2004b). Blind duplicate and triplicate blood samples were analysed in order to ensure the accuracy of the gender determination. In all cases duplicates and triplicates confirmed the results. The age of the adult birds remained unknown.
The Burrowing Parrot tends to desert its nest in response to disturbance during incubation and the first week after hatching (Masello et al. 2002). In order to reduce observer influence, nests were not disturbed until about five days after the estimated hatching date of the last nestling of a clutch. Blood sampling for gender determination had no detectable adverse effects on the birds. After measuring and sampling, the birds were released in their burrows. No desertion occurred. The number of fledglings and the pre-fledgling nestling size of handled nests were within previously reported ranges (Masello and Quillfeldt 2002, 2003, 2004a).
Feather sampling, colour measurements and analysis
The first time the adults were captured in the nest one feather from the centre of the abdominal red patch and the green-blue fourth secondary covert of the right wing were sampled for further analyses. All feathers were sampled during December. As in early studies of feathers (e.g., Cuthill et al. 1999, Langmore and Bennett 1999, Quesada and Senar 2006), we sampled feathers that were representative of the colour of the rest of the patch. The feathers of the abdominal red patch were selected for colour analyses following previous studies (see Masello and Quillfeldt 2003, 2004b, Masello et al. 2004) that revealed this patch as a conspicuous secondary sexual character signalling individual quality and parental investment. Likewise, the blue colouration of the wing feathers is very conspicuous in the Burrowing Parrot, particularly during flight. As in earlier studies (e.g., Mays et al. 2004, Moreno et al. 2007), just one representative feather of the colour was sampled in order to reduce disturbance to the birds: (1) the green-blue secondary coverts are the only feathers with blue colouration that can be easily collected without affecting flight capability, and (2) sampling of feathers of the abdominal red patch can affect the thermoregulation capability of the adult birds, which brood their nestlings overnight during the entire nestling period (Masello et al. 2006b). The secondary coverts are moulted after the end of the breeding season (January; Masello and Quillfeldt, pers. obs.). In contrast to the wing feathers, the red abdominal feathers are not moulted during the breeding season (Masello and Quillfeldt, pers. obs.), but according to aviculturists they are moulted following wing moult (C Doty and D Willis, pers. com.). Thus, all the feathers sampled for our study grew 9-11 months before sampling. Feathers may change their quality as the birds move in and out of the sandy burrows. We expect the effects of this abrasion to be homogeneous among sampled feathers, as all studied burrows belong to the same geological formation (Masello et al. 2006b) and thus, have similar physical characteristics (e.g., sand of similar granule size, humidity).
Feather reflectance was measured at the Univ. of Bristol following earlier developed procedures at the School of Biological Sciences (e.g., Langmore and Bennett 1999, Pearn et al. 2001). The distal region of the red feathers of the abdominal patch of the Burrowing Parrot is red, while the medial region is increasingly yellow and the basal region is greyish, so four measurements were taken only in the exposed part of the centre of the red region (referred to throughout this paper as ‘the red region'). The outer web of the green-blue fourth secondary coverts has a distal green region and a blue basal region (referred to throughout this paper as ‘the green region' and ‘the blue region'). Four measurements (each from a 2-mm diameter spot) were taken within the exposed part of each region. For each individual, the mean of the measure spectra was used in further analyses (see below). Avoiding the overlap of feather barbs, all feathers were carefully mounted on black velvet during measurement to eliminate stray reflections. Within feathers, regions were randomly allocated for spectrometric measurements over time, and feathers from each individual were allocated over time in a randomised block design (see Bennett et al. 1997). Feathers were illuminated from the proximal end, at 45° to the surface, using a Zeiss CLX 500 Xenon lamp. Reflected light was collected at 90° to the surface, using a Zeiss GK21 goniometer and the spectrum determined with a Zeiss MC 500 UV-VIS spectrometer. Reflectance was measured relative to a 99% Spectralon™ white standard, at a wavelength range of 300-700 nm. White references were taken between each region and between each bird, and the reflectance standard was crosschecked against a virgin standard prior to the study. Dark references were performed before each sample. We restricted spectral analyses to wavelengths from 300-700 nm, as most birds are sensitive to ultraviolet UVA wavelengths and 700 nm is likely the upper limit of the vertebrate visual spectrum (Jacobs 1981, Hill and McGraw 2006). Measurements were done blind to the gender of the individual.
Statistical procedures
Unrotated Principal Components Analyses (Sokal and Rohlf 1994) on reflectance spectral data at 2.43 nm intervals were performed separately for each feather region. Several authors (e.g., Endler 1990, Bennett et al. 1997, Cuthill et al. 1999) recommended Principal Components Analyses of reflectance spectra as an objective way of describing variation in reflectance due to the fact that principal components are independent of the visual system of humans. The Principal Components Analyses extracts three principal components for each colour region and the corresponding scores for each individual, called hereafter PC1, PC2 and PC3. The first principal component is essentially flat and therefore describes variation in mean reflectance (also termed brightness or achromatic variation, sensu Endler 1990, Bennett et al. 1997, Cuthill et al. 1999). The first principal component by this definition explains most of the between-spectra variation (Endler 1990, Endler and Théry 1996, Bennett et al. 1997). As in other studies, the second and third principal components represent variation in spectral shape (i.e., chromatic variation) and are therefore indirectly related to hue and saturation (e.g., Endler and Théry 1996, Bennett et al. 1997, Cuthill et al. 1999).
Data were analysed using SPSS 11.0. Throughout the following analyses, we used only data obtained for the first time for each bird or each breeding pair. Sample sizes for different analyses and figures varied as not all feathers were available for all colour-measured birds. For some of the analyses t-values are given in addition to F-values in order to show the direction of the studied relationships. In order to control for multiple testing, P-values were considered significant only if they were lower that a Dunn-Šidák critical P-value, calculated following the procedure described in Sokal and Rohlf (1994). Used data corresponded to 74 males and 79 females in the blue and green regions, and 76 and 77 in the red region.
Results
Figure 1 shows the mean reflectance curve for three feather regions of the Burrowing Parrot. Reflectance curves of all three regions revealed some reflection in the UV. The main variability was in the first principal component (PC1; achromatic variation) for all three colours (Table 1).

Figure 1. Mean reflectance curves of the (A) green, (B) blue, and (C) red feather regions (see text) of wild female and male individuals of Burrowing Parrot (Cyanoliseus patagonus) of north-eastern Patagonia, Argentina, and values of principal component coefficients (PC1, PC2 and PC3) from a Principal Components Analysis on reflectance spectral data plotted against wavelength.
Table 1. Sexual dichromatism in a population of Burrowing Parrot (Cyanoliseus patagonus) of north-eastern Patagonia, Argentina. The proportion of variance explained by each principal component (PC1, PC2 and PC3) from a Principal Components Analysis on reflectance spectral data for three feather regions (green, blue, and red; see text) is indicated. PC1 represents achromatic brightness; PC2 and PC3 describe most of the chromatic variation (spectral shape). Also shown are the results of three tests for sex differences: a Multivariate Analysis of Variance based on Principal Components Analysis scores, a General Lineal Model Analysis with the scores as dependent variable and sex and year as factors, and a Pair-wise t-test between the principal components of members of a breeding pair.
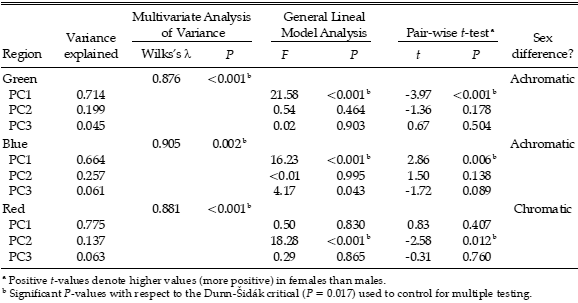
Multivariate Analyses of Variance of all adult birds considered together revealed slight (high Wilks's l) but highly significant sexual differences for all three plumage regions (Table 1). When the influence of the sampling year was taken into account in a General Lineal Model, results showed that green and blue regions of male and female differed in brightness while the red region differed in spectral shape between the sexes (Table 1).
When only breeding pair members were considered, pair-wise t-tests also demonstrated sexual differences for all three plumage regions (Table 1). In particular, males had higher achromatic brightness in the green feathers than their female partners, denoted by higher PC1 (Table 1, Fig. 1A). In contrast, the blue feathers of females had higher brightness than their male partners, denoted by higher PC1 (Table 1, Fig. 1B). The PC2 of red feathers of males were higher than those of their female partners, while there were no differences in brightness between the sexes in a pair (Table 1, Fig. 1C).
Discussion
In this study, using reflectance spectra of plumage regions, we analysed sexual dichromatism of both the structural and the psittacofulvin-based colours of wild Burrowing Parrot individuals. Although the Burrowing Parrot is not obviously dichromatic to humans (Masello et al. 2004), the present results showed slight but highly significant sexual differences in the coloration of the three plumage regions studied. While structure-based (blue) and structure-pigment-based (green) plumage regions differed in brightness, the psittacofulvin-based (red) region differed in spectral shape among the sexes. Within pairs, males had higher brightness in the green regions while females had higher brightness in the blue regions. Males of a pair had higher values of the second principal component (indirectly related to hue and saturation) in the red region, which is in accordance with previous results based only on the human-visible spectrum (Masello et al. 2004).
Plumage coloration has been found to play an important role in mate choice in many species (e.g., Bennett et al. 1997, Pearn et al. 2001, Hill and McGraw 2006). Large plumage dichromatism is usually associated with polygamous species and high levels of extra-pair paternity (e.g., Møller and Birkhead 1994, Owens and Hartley 1998), while monogamous species should display less coloration differences among the genders. Consequently, variation in the extent of sexual dichromatism among bird species is traditionally attributed to differences in social mating system. Genetically monogamous species like the Burrowing Parrot in this study (see Masello et al. 2002) are expected to display, at most, little sexual dichromatism. This is in line with the findings here reported. In this context, our results suggest mutual mate choice in the Burrowing Parrot, which is consistent with the genetic monogamy found in the species. In many monogamous birds with intense biparental care, like the Burrowing Parrot (Masello and Quillfeldt 2003, 2004a, Masello et al. 2006b), the individual characteristics (e.g., plumage coloration) of a male partner of high quality are therefore likely to be similar to those of a female partner of high quality (Jones and Hunter 1999). Some previous findings in this species (Masello and Quillfeldt 2003) provide support to our interpretation. The red abdominal patch of adult males positively correlated with male body condition and body mass suggesting that this ornament is a signal of male individual quality or foraging ability (Masello and Quillfeldt 2003). The Burrowing Parrot mated assortatively with respect to body condition and the size of the red abdominal patch (Masello and Quillfeldt 2003). That is, male parrots in good body condition and with large red ornamental patches will tend to mate with females in good body condition and with large ornamental patches (Masello and Quillfeldt 2003). Alternatively, the observed assortative mating could also be caused by genetic correlation, where the trait selected in males is also expressed in females because of gene coding. But nevertheless, additional support for our interpretation is provided by the observed condition-dependence of psittacofulvin- and structural-based coloration in the Burrowing Parrot (Masello et al. 2008). The sexual dichromatism here reported is also consistent with the slight dimorphism previously found (Masello and Quillfeldt 2003) and with previous work on the plumage coloration of this species based on methods using human colour perception (Masello et al. 2004). Regarding other Psittaciformes, slight but consistent sexual dichromatism has been reported in another recent spectrometric analysis on the Turquoise-fronted Amazon (Amazona aestiva) (Santos et al. 2006).
An interesting result of our study is the higher brightness of females in blue plumage regions compared with their partners, opposite to the typically expected pattern. Brighter plumage in females is often related to sex role reversal (Andersson 1994; but see also Heinsohn et al. 2005), which is not the case in the Burrowing Parrot, a species with intensive biparental care (Masello et al. 2006b). Our results are, however, consistent if the observed higher brightness of the blue colour in females is the less favoured state; i.e., if an overall darker blue such as observed in males is perceived as more intense as suggested in our recent study on the condition dependence of the psittacofulvin- and structural coloration (Masello et al. 2008). On a mechanistic level, work by Shawkey et al. (2003) suggests that the nanostructure of the spongy layer of blue feathers of the Eastern Bluebird (Sialia sialis) correlates with parameters of spectral shape, but not with achromatic brightness. They suggest that differences in total brightness might be caused by morphological features outside the spongy layer, or by the number of melanin granules in the barbules of the feather that likely contribute to the brightness of the feather (Shawkey et al. 2003, Shawkey and Hill 2006). It remains to be determined what causes differences in achromatic brightness of the structural colours observed in the Burrowing Parrot, a species where a spongy ultrastructure is also present (Dyck 1977).
Acknowledgements. We wish to thank Andrew Bennett for training on colour measuring methods, for providing the spectrometer and the lab facilities to conduct the colour measurements at his lab at the Univ. of Bristol. We also wish to thank Ramón Conde, Adrián Pagnossin, María L. Pagnossin, Hans-Ulrich Peter, Roberto Ure, Mara Marchesan, Tina Sommer, Mauricio Failla and Gert Dahms for their help with the fieldwork, Kate Buchanan and Anne Peters for useful comments on the manuscript and financial support, Kaspar Delhey and Roger Mundry for useful comments on the statistical analyses. This project was partially supported by the City Council of Viedma (Argentina), a grant of the state of Thuringia (Germany), a co-operation grant between the International Bureau of the BMBF of Germany (ARG 99/020) and the Argentinean SECyT (AL/A99-EXIII/003), a grant of the World Parrot Trust, several grants from the Liz Claiborne Art Ortenberg Foundation and the Wildlife Conservation Society and a grant of the British Ecological Society to JFM. We would like to thank Lisa Tell (University of California) and ZooGen Services for their assistance with the gender analysis of the 2003 blood samples. This study was carried out with permission of the Dirección de Fauna Silvestre de la Provincia de Río Negro, Argentina (Exp. no. 143089-DF-98).
Literature Cited
Andersson M (1994) Sexual selection. Princeton University Press, Princeton [ Links ]
Andersson S, Örnborg J and Andersson M (1998) Ultraviolet sexual dimorphism and assortative mating in blue tits. Proceedings of the Royal Society of London B 265:445-450 [ Links ]
Badyaev AV and Hill GE (2003) Avian sexual dichromatism in relation to phylogeny and ecology. Annual Review of Ecology, Evolution and Systematics 34:27-49 [ Links ]
Bennett ATD, Cuthill IC, Partridge JC and Lunau K (1997) Ultraviolet plumage colours predict mate preferences in starlings. Proceedings of the National Academy of Sciences 94:8618-8621 [ Links ]
Blank SM, Kutzscher C, Masello JF, Pilgrim RLC and Quillfeldt P (2007) Stick-tight fleas in the nostrils and below the tongue: evolution of an extraordinary infestation site in Hectopsylla (Siphonaptera: Pulicidae). Zoological Journal of the Linnean Society 149:117-137 [ Links ]
Burkhardt D (1989) UV vision: a bird's eye view of feathers. Journal of Comparative Physiology A 164:787-796 [ Links ]
Cabrera AL (1971) Fitogeografía de la República Argentina. Boletín de la Sociedad Argentina de Botánica 14:1-42 [ Links ]
Cuthill IC, Bennett ATD, Partridge JC and Maier EJ (1999) Plumage reflectance and the objective assessment of avian sexual dichromatism. American Naturalist 153:183-200 [ Links ]
Doucet SM and Montgomerie R (2003) Multiple sexual ornaments in satin bowerbirds: ultraviolet plumage and bowers signal different aspects of male quality. Behavioral Ecology 14:503-509 [ Links ]
Dyck J (1971a) Structure and spectral reflectance of green and blue feathers of the rose-faced lovebird (Agapornis roseicollis). Biologiske Sckrifter 18:1-67 [ Links ]
Dyck J (1971b) Structure and colour-production of the blue barbs of Agapornis roseicollis and Cotinga maynana. Zeitschrift für Zellforschung 115:17-29 [ Links ]
Dyck J (1977) Feather ultrastructure of Pesquet's Parrot Psittrichas fulgidus. Ibis 119:364-366 [ Links ]
Dyck J (1992) Reflectance of plumage areas colored by green feather pigments. Auk 109:293-301 [ Links ]
Eaton MD (2005) Human vision fails to distinguish widespread sexual dichromatism among sexually "monochromatic" birds. Proceedings of the National Academy of Sciences 102:10942-10946 [ Links ]
Endler JA (1990) On the measurement and classification of colour in studies of animal colour patterns. Biological Journal of the Linnean Society 41:315-352 [ Links ]
Endler JA and Théry M (1996) Interacting effects of lek placement, display behavior, ambient light, and color patterns in three Neotropical forest-dwelling birds. American Naturalist 148:421-452 [ Links ]
Finger E, Burkhardt D and Dyck J (1992) Avian plumage colors. Origin of UV reflection in a black parrot. Naturwissenschaften 79:187-188 [ Links ]
Heinsohn R, Legge S and Endler JA (2005) Extreme reversed sexual dichromatism in a bird without sex role reversal. Science 309:617-619 [ Links ]
Hill GE and McGraw KJ (2006) Bird coloration. Harvard University Press, Cambridge and London [ Links ]
Jacobs GH (1981) Comparative color vision. Academic Press, New York [ Links ]
Jones IL and Hunter FM (1999) Experimental evidence for mutual inter- and intrasexual selection favouring a crested auklet ornament. Animal Behaviour 57:521-528 [ Links ]
Krukenberg CFW (1882) Die federfarbstoffe der psittaciden. Vergleichend-physiologische Studien 2:29-36 [ Links ]
Langmore NE and Bennett ATD (1999) Strategic concealment of sexual identity in an estrildid finch. Proceedings of the Royal Society of London B 266:543-550 [ Links ]
Lubjuhn T and Sauer KP (1999) DNA fingerprinting and profiling in behavioural ecology. Pp. 39-52 in: Epplen JT and Lubjuhn T (eds) DNA profiling and DNA fingerprinting. Birkhäuser Verlag, Basel [ Links ]
Lubjuhn T, Sramkova A, Masello JF, Quillfeldt P and Epplen JT (2002) Truly hypervariable DNA fingerprints due to exceptionally high mutation rates. Electrophoresis 23:517-519 [ Links ]
Mahler BA and Kempenaers B (2002) Objective assessment of sexual plumage dichromatism in the Picui Dove. Condor 104:248-254 [ Links ]
Masello JF, Choconi RG, Helmer M, Kremberg T, Lubjuhn T and Quillfeldt P (2009) Do leucocytes reflect condition in nestling burrowing parrots (Cyanoliseus patagonus) in the wild? Comparative Biochemistry and Physiology A 152:176-181 [ Links ]
Masello JF, Choconi RG, Sehgal RMN, Tell LA and Quillfeldt P (2006a) Blood and intestinal parasites in wild Psittaciformes: a case study of Burrowing Parrots (Cyanoliseus patagonus). Ornitología Neotropical 17:515-529 [ Links ]
Masello JF, Lubjuhn T and Quillfeldt P (2008) Is the structural and psittacofulvin-based colouration of wild Burrowing Parrots Cyanoliseus patagonus condition dependent? Journal of Avian Biology 39:653-662 [ Links ]
Masello JF, Pagnossin GA, Palleiro GE and Quillfeldt P (2001) Use of miniature security cameras to record behaviour of burrow-nesting birds. Vogelwarte 41:150-154 [ Links ]
Masello JF, Pagnossin ML, Lubjuhn T and Quillfeldt P (2004) Ornamental non-carotenoid red feathers of wild Burrowing Parrots. Ecological Research 19:421-432 [ Links ]
Masello JF, Pagnossin ML, Sommer C and Quillfeldt P (2006b) Population size, provisioning frequency, flock size and foraging range at the largest known colony of Psittaciformes: the Burrowing Parrots of the north-eastern Patagonian coastal cliffs. Emu 106:69-79 [ Links ]
Masello JF and Quillfeldt P (2002) Chick growth and breeding success of the Burrowing Parrot. Condor 104:574-586 [ Links ]
Masello JF and Quillfeldt P (2003) Body size, body condition and ornamental feathers of Burrowing Parrots: Variation between andears and sexes, assortative mating and influences on breeding success. Emu 103:149-161 [ Links ]
Masello JF and Quillfeldt P (2004a) Consequences of La Niña phase of ENSO for the survival and growth of nestling Burrowing Parrots on the Atlantic coast of South America. Emu 104:337-346 [ Links ]
Masello JF and Quillfeldt P (2004b) Are haematological parameters related to body condition, ornamentation and breeding success in wild burrowing parrots Cyanoliseus patagonus? Journal of Avian Biology 35:445-454 [ Links ]
Masello JF and Quillfeldt P (2008) Klimawandel und brutverhalten: erfolgreich brüten in wechselhafter umwelt? Eine fallstudie am felsensittich. Vogelwarte 46:302-303 [ Links ]
Masello JF, Sramkova A, Quillfeldt P, Epplen JT and Lubjuhn T (2002) Genetic monogamy in burrowing parrots Cyanoliseus patagonus? Journal of Avian Biology 33:99-103 [ Links ]
Mays HL, McGraw KJ, Ritchison G, Cooper S, Rush V and Parker RS (2004) Sexual dichromatism in the andellow-breasted chat Icteria virens: spectrophotometric analysis and biochemical basis. Journal of Avian Biology 35:125-134 [ Links ]
McGraw KJ and Nogare MC (2004) Carotenoid pigments and the selectivity of psittacofulvin-based coloration systems in parrots. Comparative Biochemistry and Physiology B 138:229-233 [ Links ]
McGraw KJ and Nogare MC (2005) Distribution of unique red feather pigments in parrots. Biology Letters 1:38-43 [ Links ]
Mey E, Masello JF and Quillfeldt P (2002) Chewing lice (Insecta, Phthiraptera) of the Burrowing Parrot Cyanoliseus p. patagonus (Vieillot) from Argentina. Rudolstädter Naturhistorische Schriften, Supplement 4:99-112 [ Links ]
Møller AP and Birkhead TR (1994) The evolution of plumage brightness in birds is related to extra-pair paternity. Evolution 48:1089-1100 [ Links ]
Morelli R, Loscalzo R, Stradi R, Bertelli A and Falchi M (2003) Evaluation of the antioxidant activity of new carotenoid-like compounds by electron paramagnetic resonance. Drugs under Experimental and Clinical Research 29:95-100 [ Links ]
Moreno J, Merino S, Lobato E, Rodríguez-Gironés MA and Vásquez RA (2007) Sexual dimorphism and parental roles in the thorn-tailed rayadito (Furnariidae). Condor 109:312-320 [ Links ]
Owens IPF and Hartley IR (1998) Sexual dimorphism in birds: why are there so many different forms of dimorphism? Proceedings of the Royal Society of London B 265:397-407 [ Links ]
Pearn SM, Bennett ATD and Cuthill IC (2001) Ultraviolet vision, fluorescence and mate choice in a parrot, the budgerigar Melopsittacus undulatus. Proceedings of the Royal Society of London B 268:2273-2279 [ Links ]
Pearn SM, Bennett ATD and Cuthill IC (2003) The role of ultraviolet A-reflectance and ultraviolet A-induced fluorescence in the appearance of budgerigar plumage: insights from spectrofluorometry and reflectance spectrophotometry. Proceedings of the Royal Society of London B 270:859-865 [ Links ]
Prum RO and Torres R (2003) Structural colouration of avian skin: convergent evolution of coherently scattering dermal collagen arrays. Journal of Experimental Biology 206:2409-2429 [ Links ]
Prum RO, Torres R, Williamson S and Dyck J (1999) Two-dimensional Fourier analysis of the spongy medullary keratin of structurally coloured feather barbs. Proceedings of the Royal Society of London B 266:13-22 [ Links ]
Quesada J and Senar JC (2006) Comparing plumage colour measurements obtained directly from live birds and from collected feathers: the case of the great tit Parus major. Journal of Avian Biology 37:609-616 [ Links ]
Santos SICO, Elward B and Lumeij JT (2006) Sexual dichromatism in the Blue-fronted Amazon Parrot (Amazona aestiva) revealed by multiple-angle spectrometry. Journal of Avian Medicine and Surgery 20:8-14 [ Links ]
Shawkey MD, Estes AM, Siefferman LM and Hill GE (2003) Nanostructure predicts intraespecific variation in ultraviolet-blue plumage colour. Proceedings of the Royal Society of London B 270:1455-1460 [ Links ]
Shawkey MD and Hill GE (2005) Carotenoids need structural colours to shine. Biology Letters 1:121-124 [ Links ]
Shawkey MD and Hill GE (2006) Significance of a basal melanin layer to production of non-iridescent structural plumage color: evidence from an amelanotic Steller's jay (Cyanocitta stelleri). Journal of Experimental Biology 209:1245-1250 [ Links ]
Sokal RR and Rohlf FJ (1994) Biometry. The principles and practice of statistics in biological research. Third edition. Freeman and Company, New York [ Links ]
Stradi R, Pini E and Celentano G (2001) The chemical structure of the pigments in Ara macao plumage. Comparative Biochemistry and Physiology B 130:57-63 [ Links ]
Tubaro PL, Lijtmaer DA and Lougheed SC (2005) Cryptic dichromatism and seasonal color variation in the diademed tanager. Condor 107:648-656 [ Links ]
Veronelli M, Zerbi G and Stradi R (1995) In situ resonance raman spectra of carotenoids in birds' feathers. Journal of Raman Spectroscopy 26:683-692 [ Links ]
Völker O (1936) Über den gelben federfarbstoff des Wellensittichs (Melopsittacus undulatus (Shaw)). Journal für Ornithologie 84:618-630 [ Links ]
Völker O (1937) Über fluoreszierende, gelbe federpigment bei papageien, eine neue klasse von federfarbstoffen. Journal für Ornithologie 85:136-146 [ Links ]
Völker O (1942) Die gelben und roten federfarbstoffe der papageien. Biologisches Zentralblatt 62:8-13 [ Links ]














