Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
El hornero
Print version ISSN 0073-3407On-line version ISSN 1850-4884
Hornero vol.31 no.2 Ciudad Autónoma de Buenos Aires Dec. 2016
ARTÍCULO
Long-term association of Tyrannus savana and Sturnella superciliaris density with land cover and climatic variables in agroecosystems of Argentina
Noelia C. Calamari 1,4, Alexis Cerezo Blandón2, Sonia B. Canavelli 1, Sebastián Dardanelli 1, Gregorio I. Gavier-Pizarro 3 y María E. Zaccagnini 3
1 Estación Experimental Agropecuaria Paraná, Instituto Nacional de Tecnología Agropecuaria (INTA). Ruta 11, km 12.7, 3101 Oro Verde, Entre Ríos, Argentina.
2 Fundación para la Conservación y el Ecodesarrollo (FUNDAECO). 25 calle 2-39, Zona 1, 0101 Ciudad de Guatemala, Guatemala.
3 Instituto de Recursos Biológicos, Instituto Nacional de Tecnología Agropecuaria (IRB-CNIA-INTA). De los Reseros y Las Cabañas s/n, B1712WAA Hurlingham, Buenos Aires, Argentina.
4 calamari.noelia@inta.gob.ar
ABSTRACT
Agricultural expansion and intensification resulted in important changes in the agricultural landscape of the Pampas region, Argentina. We used linear mixed models to analyze the relationship between environmental variables associated to land use/cover, productivity and climate and changes in densities of two bird species, the Fork-tailed Flycatcher (Tyrannus savana) and the White-browed Blackbird (Sturnella superciliaris). The study area in central Argentina was divided in three agroproductive sub-regions: (1) predominantly agricultural, (2) mixed agricultural- rangeland and (3) mixed agricultural-forested rangeland. Bird populations were sampled annually during 2003-2011 using point-transects along secondary roads (48 transects). Mean estimated density for Fork-tailed Flycatcher was 0.132 ind/ha, increased in the agricultural sub-region and was associated with decreasing forest cover and increasing fallow and weedy fields cover. Mean density of the White-browed Blackbird was 0.045 ind/ha, decreased in the agricultural-rangeland sub-region and increased in landscapes with more perennial pastures, fallow and weedy fields and annual pastures, avoiding sites with more forest cover. Productivity and climatic variables only showed a strong association with White-browed Blackbird density. Our results suggest that land use/cover, productivity and climatic factors are important variables when planning strategies to conserve bird population at a regional level in agroecosystems of Argentina.
KEY WORDS: Argentina; Biological monitoring; Bird population trends; Density; EVI; Land use; Rainfall; Temperature.
RESUMEN
Asociación a largo plazo de la densidad de Tyrannus savana y Sturnella superciliaris con variables de cobertura de la tierra y climáticas en agroecosistemas de Argentina.
La expansión e intensificación agrícolas resultaron en importantes cambios en el paisaje agrícola de la Región Pampeana Argentina. Se utilizaron modelos lineales generalizados mixtos para analizar la relación entre la densidad de dos especies de aves, la Tijereta (Tyrannus savana) y el Pecho Colorado (Sturnella superciliaris), y variables ambientales asociadas a la cobertura, los usos de la tierra, la productividad vegetal y las condiciones climáticas. El área de estudio en la región central de Argentina se dividió en tres subregiones: (1) predominantemente agrícola, (2) agrícola-ganadera y (3) agrícola-ganadera bajo bosque nativo. Las aves fueron muestreadas anualmente durante 2003-2011 en 48 transectas de puntos localizadas en caminos secundarios. La densidad promedio de la Tijereta fue de 0.132 ind/ha, se incrementó significativamente en la subregión agrícola, y estuvo asociada a la disminución en la cobertura de bosque y al incremento en la proporción de cobertura de rastrojo y campo en descanso. La densidad del Pecho Colorado fue de 0.045 ind/ha, decreció en la subregión agrícola-ganadera y se incrementó en paisajes con mayor proporción de pasturas perennes, cobertura de rastrojo, campos en descanso y pasturas anuales, evitando sitios con mayor cobertura de bosque. La productividad y las variables climáticas solamente mostraron una asociación fuerte con la densidad del Pecho Colorado. Nuestros resultados sugieren que la cobertura de usos de la tierra, la productividad y los factores climáticos son importantes al planificar estrategias de conservación de aves a escala regional en los agroecosistemas de Argentina.
PALABRAS CLAVE: Argentina; Densidad; EVI; Monitoreo biológico; Precipitaciones; Temperatura; Tendencia poblacional de aves; Uso de la tierra.
Received 28 January 2016
Accepted 29 December 2016
The simplification of agricultural landscapes, associated with habitat loss and agricultural intensification, has been shown to reduce the availability of resources for many bird species with specific requirements (Benton et al. 2003, Vickery et al. 2009, Guerrero et al. 2012) and, consequently, the abundance of these species (Chamberlain et al. 2000, Donald et al. 2006). The decreases on bird abundance are related to local changes in vegetation cover, structure and diversity (Heikkinen et al. 2004, Filloy and Bellocq 2007). Simplification of agricultural landscapes also produces changes in temporal population dynamics of bird species (Boulinier et al. 1998, 2001).
Species may respond differently to land use and climatic factors (Firbank et al. 2008, Karsh and MacIver 2009, Concepción and Díaz 2010). Some species may be negatively affected, eliciting negative population trends, and some others may be positively affected, with resulting increases in their population abundance (Holt 2003, Both and te Marvelde 2007, Angert 2009, Both et al. 2010). However, some other species may not be affected and maintain their abundance relatively stable, at least in the short term (Siriwardena et al. 1998, Fewster et al. 2000), because they may have been able to exploit alternative food supplies or search for food over a wide area for a period of time (Chamberlain et al. 2000). In any case, bird responses are thought to be determined by life-history characteristics such as regional abundance or the tolerance range to environmental factors (climatic, topographic or biological factors; Brown and Lomolino 1998, Newbold et al. 2013, Bradshaw et al. 2014, Bregman et al. 2014). Consequently, an important challenge for biodiversity conservation is to determine species responses to annual variations in land use and climatic conditions, in order to improve assessments of impacts and risks and thus develop appropriate mitigation strategies. Interannual variations in climate is another factor known to influence reproduction and survival of songbirds (Newton 1998). In particular, temperature and precipitation can influence directly bird survival and breeding success via inclement weather and indirectly through its effects on food abundance (Newton 1998, Morrison and Bolger 2002, Wilson et al. 2011).
During the last half-century (1950-2000), the increasing worldwide demand for food, important key technological advances in the agricultural sector, and high national and international competitiveness, have triggered agricultural expansion and intensification in regions of Argentina such as the Pampas, Espinal and Chaco (Viglizzo et al. 2004, Grau et al. 2005, Zak et al. 2008, Gasparri and Grau 2009). In these regions, native cover types (grasslands and forests) have been extensively replaced by annual crops (Viglizzo et al. 1997, Paruelo et al. 2005, Baldi and Paruelo 2008). Additionally, the excessive and extensive use of agrochemicals, soil degradation and habitat loss and fragmentation, have strongly degraded remaining original vegetation (Zaccagnini and Calamari 2001, Paruelo et al. 2005, Boletta et al. 2006, Baldi and Paruelo 2008, Codesido et al. 2008, Oesterheld 2008, Gasparri and Grau 2009). Consequently, habitat quality for resident biota has been affected, influencing the abundance and persistence of birds on those regions (Fernández et al. 2003, Filloy and Bellocq 2007, Codesido et al. 2008, Cerezo et al. 2011, Gavier-Pizarro et al. 2012, Macchi et al. 2013).
Given that incorporation of new lands to agricultural production on those regions is expected to continue in the following decades (Zak et al. 2008, Baldi and Paruelo 2008, Nori et al. 2012), potentially affecting habitat quantity and quality for avian populations, it is important to determine bird population status and to evaluate the influence of environmental factors on bird populations at a regional level for developing management and conservation strategies for birds and their habitats. A previous study conducted in central Argentina showed that relationships between bird densities and land use types depended on bird feeding guild, with insectivorous birds relying either in annual crop area or non-plowed fields, and granivorous birds having a weak relationship with land use (Gavier-Pizarro et al. 2012). However, the study only evaluated these relationships using data from 2007-2009, trading space for time through a gradient of land use/cover transformation from totally transformed areas to those dominated by natural vegetation, but did not evaluate temporal trends. Variations in environmental factors in agroecosystems (e.g., diminished food supplies as a result of climatic events, less suitable nesting habitat as a result of land use change) and their influence on bird popula tion assessed during short-term periods would not reflect a potential time lag induced by the life history of species, which can be observed in long-term studies (Chamberlain et al. 2000, Bennet et al. 2006, Cueto et al. 2008).
In this study, we explored bird-habitat relationships for two common insectivorous bird species from the Neotropics (Stotz et al. 1996), the Fork-tailed Flycatcher (Tyrannus savana) and the White-browed Blackbird (Sturnella superciliaris), during the austral breeding season (Fraga 2016, Mobley and Garcia 2016). The Fork-tailed Flycatcher is an austral migrant species member of the South American Temperate-Tropical migratory system (SATT; Joseph 1997, Jahn et al. 2013). It inhabits open habitats, preferring savannas and pastures with scattered trees and bushes where this species builds nests (Mobley and Garcia 2016). White-browed Blackbird is a resident species distributed along central-east South America, where it prefers lush wet meadows and humid grasslands, nowadays particularly common in agricultural fields or pastures with wheat, oat, alfalfa and others (Fraga 2016). This species nest on the ground within grasslands, pastures or crops (Fraga 2016). The study extended for nine years, from 2003-2011. The consideration of a longer time span compared to the previous study (three years in Gavier- Pizarro et al. 2012) allowed us to evaluate bird population trends and spatial patterns of birdhabitat relationships over time, within specific sub-regions. This more comprehensive analysis would allow us to corroborate or refute short-term bird-habitat relationships found in Gavier-Pizarro et al. (2012). Knowledge of population trajectory and status of the Forktailed Flycatcher and the White-browed Blackbird and their associations with environmental factors at a regional scale on a longterm span also could be useful to support conservation initiatives for these species.
METHODS
Study area The study area comprised 128200 km2 of the Pampas and Espinal ecoregions (Cabrera 1976) in central Argentina (Fig. 1). Temperatures in the area vary between 13 °C (annual mean minimum) and 23 °C (annual mean maximum) (Soriano et al. 1991), and mean annual precipitation is about 1000 mm (Brescia et al. 1998, Messina et al. 1999). A portion of the area corresponds to the Espinal ecoregion, which extends from the center of Santa Fe Province to northeastern Córdoba and northern Entre Ríos provinces (Fig. 1). The Espinal is characterized by remnants of xerophytic forest dominated by species such as Prosopis spp., Acacia spp., Celtis ehrenbergiana and Geoffroea decorticans, immersed in an agricultural matrix (Lewis et al. 2009, Matteucci 2012a). Another portion of the area corresponds to the Pampas ecoregion, which extends from southern Santa Fe Province to southeastern Córdoba and southern Entre Ríos provinces (Fig. 1). The Pampas were originally characterized by grasslands dominated by Stipa spp., Bromus spp. and Poa spp. (Cabrera 1971, Matteucci 2012b). However, grasslands have been modified and in many cases replaced by agricultural and cattle production activities (Matteucci 2012b). Agricultural crops cover the largest percentage of the area, including soybean, wheat, corn, sunflower and sorghum, in order of importance.

Figure 1. Map of the study area showing the productive sub-regions and location of sampling transects along secondary and tertiary roads in agroecosystems of central Argentina. The black line separates Espinal (north) and Pampa (south) ecoregions (adapted from Matteucci 2012a).
Sampling design
The area was stratified into three subregions, based on the predominant production activity: (1) agriculture (agricultural sub-region), (2) mixed agriculture and cattleranching activities (agricultural-rangeland), and (3) mixed agriculture and cattle-ranching activities with forest understories incorporated into cattle rangelands (agricultural- forested rangeland). This stratification was made based on the 1993 national agricultural and livestock survey, in which the central region of Argentina was divided into zones according to dominant productive activities (INDEC 1995). Additionally, we placed a grid with a cell size of 30×30 km over the complete study area using a Geographical Information System. In each sub-region, we selected grid cells using a systematic design (one of every two) with a random start (Canavelli et al. 2003, 2004). On each cell, we randomly selected a secondary or tertiary road as a survey route for bird observation.
Each survey route (48 in total) contained 30 points, separated by a distance of 1 km. On each point, the abundance of bird species was recorded during 3 min using distance sampling methods (Bibby et al. 2000, Buckland et al. 2001). Routes were sampled once a year (January) during 2003-2011, and between 06:00-11:00 or 15:00-20:00 h, by two experienced observers previously trained in the field sampling protocol. January was selected for field sampling due to biological reasons, including breeding season and presence of migratory species, potential greater risks of birds to agrochemical exposure and, finally, for logistic reasons (extended observation period due to sunlight hours). Further sampling details can be found in Canavelli et al. (2003) and Zaccagnini et al. (2010).
Estimation of bird density
Density for each species was estimated using the software Distance (version 5.0; Buckland et al. 2001, Thomas et al. 2002). We first conducted exploratory analyses to detect and correct the presence of clustering, evasive movements and extreme values (Buckland et al. 2001, Thomas et al. 2002). We then truncated and eliminated data for both species beyond 250 m of each observation (90% of detections were made within this distance). Additionally, we manually defined seven distance classes (0-30, 30-50, 50-75, 75-100, 100-150, 150-200 and 200-250 m) to improve data organization and model adjustment to data.
Species density was estimated using a combination of three models (uniform, halfnormal and hazard rate), and two adjustment terms (cosine and polynomial). Model fit was evaluated using q-q plots, Kolmogorov- Smirnov and Cramer-von Mises tests, as well as visual analysis of the detection probability function. The most parsimonious models were selected using the Akaike Information Criterion (AIC; Akaike 1974, Buckland et al. 2001, Burnham and Anderson 2002). The AIC selection considers the fit as well as the complexity of the model, and allows comparison of several models simultaneously (Johnson and Omland 2004). The AIC values reflect the amount of “information” lost when a model is used to approximate conceptual reality; thus, the model with the lowest AIC value is selected as the best model (Burnham and Anderson 1998).
The half-normal model with a cosine adjustment was selected for both species based on its consistently lower AIC value. For each species, we estimated the detection probability function globally (i.e., combining all years) and bird density for each route and year by stratifying per year and post-stratifying per route, with conventional distance sampling procedures (Buckland et al. 2001).
Environmental variables
Many studies have postulated that intensification of agriculture through land use changes or climatic factors may have contributed to farmland bird decline (Chamberlain et al. 2000, Benton et al. 2003, Lemoine et al. 2007, Cueto et al. 2008, Niven et al. 2009). Although it is known that these factors have an impact on birds, it is unclear which is currently most important for the Fork-tailed Flycatcher and the White-browed Blackbird at regional and sub-regional scales. Climatic variables used in models were temperature (annual mean minimum and maximum) and rainfall (four-month average; see below) (Table 1). Temperature and rainfall were calculated for each route, taken from the nearest meteorological station of the National Institute of Agricultural Technology (INTA). Given that the bird breeding season in central Argentina extends from September to January, we averaged monthly rainfall over this four-month period for each year, with the assumption that species abundance in January will be influenced by rainfall in the four previous months to the sampling period (first days of January). This relationship could be based on the food availability for bird species, given that phenology of plants and insects observed in January can be associated with rainfall occurred in previous months (De Juana and García 2005). It also could be related to bird’s breeding performance, because climatic conditions can influence the metabolism of bird females and also directly affect egg and chick survival (Barkowska et al. 2003, Dunn 2004).
Table 1. Description of predictive variables included in statistical models of bird-habitat relationship for the Fork-tailed Flycatcher (Tyrannus savana) and the White-browed Blackbird (Sturnella superciliaris) in agroecosystems of central Argentina.

Some bird species depend on particular environmental conditions along land use/ cover gradients and primary productivity could have an influence (Fischer et al. 2006, Schrag et al. 2009). Primary productivity, a measure of the energy entering an ecosystem, was measured using the Enhanced Vegetation Index (EVI; Table 1). We selected this index instead of NDVI (Normalized Difference Vegetation Index), due to EVI is more responsive to canopy structural variations, including leaf area index, canopy type, plant physiognomy, and canopy architecture (Gao et al. 2000, Huete et al. 2002). EVI was developed to optimize the vegetation signal, improving sensitivity in high biomass regions and, therefore, improving vegetation monitoring through a decoupling of the canopy background signal and climatic influences (Matsushita et al. 2007, Jiang et al. 2008). For each route per year, we extracted EVI values from a MODIS satellite image (mid-January) with a 250 m resolution (EOS-Terra images/MODIS, mid-January of each year). An EVI value was estimated for each 250×250 m pixels centered on the midpoint location of each route. Then, EVI values were rescaled by dividing the values by 10 000, assuming value in a 0-1 range. Low value areas indicate scarce or no vegetation while high value areas indicate vigorous vegetation.
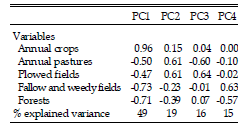
Land use/cover variables were obtained from the field, based on the proportional cover of land use classes visually estimated in a 200 m radius circle around each sampling point. The land use classes (each representing a variable) were: annual crops (corn, soybean, sunflower and sorghum-summer crops), annual pastures (millet, oats), perennial pastures (alfalfa, clover), plowed fields (i.e., bare soil), fallow and weedy fields, and forests (native, introduced, or mixed). To have a unique value of proportional cover for each class at the route level, we averaged the proportion of each cover class on each point over all the observations points in a route. Variables of vegetation cover were highly correlated (r ≥ 0.7, except for perennial pastures cover), making difficult the interpretation of relationships between individual variables and bird abundance. Therefore, we used Principal Components Analysis (PCA; McCune et al. 2002) to obtain independent measures of land use/cover variables (Table 1). PCA is a multivariate ordination technique which represents a data set containing many variables (in this case, vegetation cover variables) with a smaller number of composite or latent variables (the components or axes of the PCA; Graham 2003, Dormann et al. 2013). These axes are orthogonal (i.e., they are completely uncorrelated), and represent the strongest covariation patterns among the variables in the original data set (McCune et al. 2002). The first four PCA components represented 49, 19, 16 and 15% of the total variance in the original data matrix and were at least moderately correlated (r ≥ 0.4) with at least one land cover variable (Table 2). Given the value and sign of correlations of the first component (PC1) with the original variables, this axis represented a gradient from sites with low annual crops cover and high forests and fallow and weedy fields cover to sites with high annual crops cover and low forests and fallow and weedy fields cover. The second component (PC2) represented a gradient of covariation between plowed fields and annual pastures (i.e., a gradient of sites with low to high values of both variables). The third component (PC3) represented a gradient of sites with high values of plowed fields cover and low values of annual pastures cover and sites with low values of plowed fields cover and high values of annual pastures cover. Finally, the fourth component (PC4) represented a gradient of sites with low forests cover and high cover of fallow and weedy fields to sites with high forests cover and low proportional cover of fallow and weedy fields. In order to evaluate the potential effects of multicollinearity, we calculated Spearman correlation coefficients between all candidate variables. Spearman correlations were generally low (rs ≤ 0.30), and thus we assumed that our results were not significantly affected by multicollinearity.
Table 2. Results of a Principal Components Analysis of land use/cover variables recorded on sampling transects along secondary and tertiary roads in agroecosystems of central Argentina. Correlations between components and variables are shown.
Species density-environment models
Because of the nested structure of the data (points within routes, routes within sampling periods, sampling periods within observers), we used linear mixed modelling to analyse the relationship between the estimated density for each bird species and annual environmental variables (Pinheiro and Bates 2000). In all models, bird density was the dependent variable. The fixed effects (i.e., independent or predictive variables) were the year (to test for a temporal trend) and the environmental variables describing climatic, primary productivity and land use/cover variation (Table 1). All predictive variables were standardized to vary between 0 and 1, to be able to compare model coefficients directly. Random effects were observer, route and sampling period (morning or evening). Also, sub-region was used as a random effect for models that used the complete, regional data set. Bird density data was log-transformed to meet the statistical assumption of a normal error distribution. In general, model errors did not meet the variance homogeneity assumption, and we re-fitted models with a heterogeneous variance structure between years (Pinheiro and Bates 2000). Finally, because of the spatial nature of our sampling scheme, we also fitted models with a spatial autocorrelation error structure. Because of the aforementioned features associated to our data set, we fitted models with four different structures, for each species and sub-region, and for the complete study region (all sub-regions combined): (1) without random effects, assuming homogeneity of variances and independence between errors (standard multiple regression model); (2) with random effects and heterogeneity of variances between years; (3) with random effects and homogeneity of variances between years; and (4) without random effects and assuming homogeneity of variances, but with a temporal and spatial autocorrelation structure, to test for lack of independence between errors (Table 3). We used AIC values to select among these different model structures; the used model structure was the one with the lowest AIC value (Table 3). All analyses were conducted in R software with the nlme (Pinheiro et al. 2013) and lattice (Sarkar 2008) packages.
Table 3. Comparison of the structure of statistical models of bird-habitat relationship for the Fork-tailed Flycatcher (Tyrannus savana) and the White-browed Blackbird (Sturnella superciliaris) in agroecosystems of central Argentina. AIC values for each model structure in the complete study region (CSR) and in the agricultural-rangeland (AR), agricultural-forested rangeland (AFR) and agricultural (A) productive subregions are shown. NC: non-convergence.

Once the optimal model structure was found, best-fitting models were selected using a combination of traditional hypothesis testing and Kulback-Leibler Information methods, particularly AIC values and Akaike weights (wi) (Burnham and Anderson 1998, 2001, Anderson et al. 2000). For each bird species, we initially fitted a set of 15 models with different combinations of year (i.e., linear temporal trend), land use/cover, EVI, and climatic variables (Table 4). We used this exploratory approach, rather than a more restricted set of a priori models (Burnham and Anderson 1998, 2002), because we had no prior information on the explanatory power of our variables in combination. Then, for each model, we examined the P-values for each predictive variable in the set, selected variables with P ≤ 0.20, and re-fitted the model using only those variables. The number of fitted models for each species varied between 22 for Fork-tailed Flycatcher in the agricultural-rangeland sub-region and 24 for White-browed Blackbird in agriculturalforested rangeland sub-region. Finally, we used AIC and wi values to choose the bestfitting models from the final set of fitted models (Table 5). When differences between AIC values were small (≤ 2 units), wi values were used as indicators of the strength of evidence for each model. The wi is interpreted as the approximate probability that model i is the best model in the set of models being considered (Anderson et al. 2000).
Table 4. Initial 15 models of bird-habitat relationship for the Fork-tailed Flycatcher (Tyrannus savana) and the White-browed Blackbird (Sturnella superciliaris) in agroecosystems of central Argentina fitted to data. Predictive variables included in models are described in table 1.
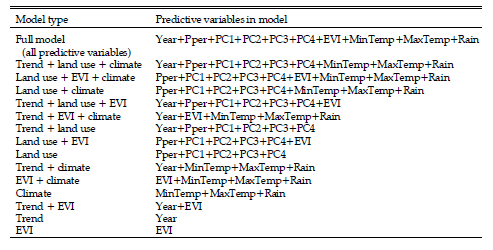
Table 5. Confidence set of models of bird-habitat relationship for the Fork-tailed Flycatcher (Tyrannus savana) and the White-browed Blackbird (Sturnella superciliaris) in agroecosystems of central Argentina. AIC and wi values for each model in the complete study region (CSR) and in the agricultural- rangeland (AR), agricultural-forested rangeland (AFR) and agricultural (A) productive sub-regions are shown. Predictive variables included in models are described in table 1.

We used multi-model inference or model averaging (Burnham and Anderson 2002) to compare the effect size of individual predictive variables. Model averaging consists in obtaining an averaged coefficient value from several models, weighted by each model’s wi (Burnham and Anderson 1998). We obtained an averaged coefficient for each predictive variable from the set of models with a sum of wi ≥ 0.95 (i.e., the confidence model set). The number of models in confidence sets was much smaller than the complete set of models for each bird species, varying between two models for Fork-tailed Flycatcher in the complete study region and the agriculturalrangeland sub-region and six models for the White-browed Blackbird in the agriculturalforested rangeland sub-region. Overall mean (±SD) number of models in confidence sets was 4.4±2.4.
RESULTS
Trends in population density
Fork-tailed Flycatcher mean (±SE) density was approximately three times higher than the density of the White-browed Blackbird (0.132±0.01 and 0.045±0.01 individuals/ha, respectively; Fig. 2). Population density of the Fork-tailed Flycatcher showed statistically significant changes only in the agricultural subregion, were it increased, while the density of the White-browed Blackbird significantly decreased only in the agricultural-rangeland sub-region (Fig. 2). However, both trends were probably influenced by outlier values, such as the unusually high density of the Fork-tailed Flycatcher in 2008 and White-browed Blackbird in 2003 (Fig. 2).

Figure 2. Variation in density (individuals/ha) of the Fork-tailed Flycatcher (Tyrannus savana) and the White-browed Blackbird (Sturnella superciliaris) in agroecosystems of central Argentina during 2003- 2011. Mean (±SE) values for each species in the complete study region and in the agricultural-rangeland, agricultural-forested rangeland and agricultural productive sub-regions are shown.
Annual changes in environmental variables
Vegetation productivity and climatic variables did not show statistically significant annual changes in the analysed period either on the complete study region or the sub- regions. However, land use variables showed temporal trends on some cases (Table 6). Annual crops showed a negative trend in the complete study region and in the agricultural and agricultural-rangeland sub-regions. Additionally, perennial pastures showed a negative trend in the complete study region and in the agricultural sub-region. Finally, fallow and weedy fields presented a negative trend in the agricultural-forested rangeland sub-region but a positive trend in the agricultural sub-region (Table 6).
Table 6. Mean (±SE) values of the regression coefficient (â), 95% confidence intervals and associated statistics for land use cover variables recorded on sampling transects along secondary and tertiary roads in agroecosystems of central Argentina that showed a significant temporal trend.

Bird density and environmental variables
Bird density was related to environmental variables to different degrees, depending on the species and sub-regions. The density of the Fork-tailed Flycatcher increased in landscapes characterized by decreasing forest cover and increasing fallow and weedy fields in the complete study region and in all subregions (positive relationship to PC4; Figs. 3A-D). Additionally, the density decreased with increasing plowed fields cover and decreasing annual pastures, but only in the agricultural- forested rangeland sub-region (negative association to PC3; Fig. 3C). In the agricultural- rangeland sub-region, although the Fork-tailed Flycatcher increased in landscapes with high proportion of annual crops cover and decreased in sites with low fallow and weedy fields and forest cover (positive association with PC1; Fig. 3B), it was still strongly associated to the fourth component, and this association was higher than its association to PC1. Thus, its negative association to PC1 (and thus to its usually preferred cover types, low fallow and weedy fields) was probably asso ciated to its avoidance of forest which, on PC1, was associated to fallow and weedy fields. In the agricultural sub-region, however, its relationship to PC1 was inverted to that shown in the agricultural-rangeland sub-region. Perhaps in the agricultural region, the amount of fallow and weedy fields is higher or forest cover is much lower, thus making these landscapes more attractive. Climatic variables were weakly associated: the density of the Forktailed Flycatcher showed a positive relationship with rainfall in the agricultural- rangeland sub-region (Fig. 3B) and was also weakly and negatively related to annual minimum temperature in the agricultural sub-region (Fig. 3D).

Figure 3. Mean (±SE) values of coefficients (adjusted by Akaike weights) for all predictive variables included in statistical models of bird-habitat relationship for the Fork-tailed Flycatcher (Tyrannus savana) and the White-browed Blackbird (Sturnella superciliaris) in agroecosystems of central Argentina. Values for the complete study region and for the agricultural-rangeland, agricultural-forested rangeland and agricultural productive sub-regions are shown. Predictive variables are described in table 1.
The density of the White-browed Blackbird increased with decreasing fallow and weedy fields and forest cover and increasing annual crops cover (positive association to increases in PC1) in the complete study region (Fig. 3E) and in the agricultural sub-region (Fig. 3H). Additionally, it increased in landscapes with high proportion of annual pastures and plowed fields (positive associations to PC2), and increased in landscapes with low proportion of forest cover and high cover of fallow and weedy fields (positive associations to PC4) in the complete study region (Fig. 3E) and in the agricultural-rangeland (Fig. 3F) and the agricultural (Fig. 3H) sub-regions. Its density was also positively associated with perennial pastures, both in the complete region and in all sub-regions (Figs. 3E-H). Furthermore, White-browed Blackbird decreased with increasing plowed fields and decreasing annual pastures cover (negative relationship to PC3) in the complete study region (Fig. 3E) and in the agricultural-rangeland sub-region (Fig. 3F). In contrast to the density of the Forktailed Flycatcher, climatic variables and vegetation productivity were more strongly associated with the density of the Whitebrowed Blackbird. It showed a positive relationship with rainfall and EVI but decreased with annual maximum temperature in the complete study region (Fig. 3E) and in most of the sub-regions. In sharp contrast to its more complex response in the agricultural- rangeland and agricultural sub-regions, where it responded to multiple environmental variables, this species only responded to EVI in the agricultural-forested rangeland sub-region (Fig. 3G).
DISCUSSION
Land use/cover and productivity were the main factors explaining the long-term population changes of the Fork-tailed Flycatcher and the White-browed Blackbird in agroecosystems of central Argentina. These results strenghten the weak relationships found in the previous short-term study (Gavier-Pizarro et al. 2012), highlighting the importance of monitoring long-term population trends (Magurran et al. 2010).
Different bird responses to landscape changes may be relate to degrees of specialization to landscape elements (Andrén et al. 1997). In our study, both species responded mainly to evolving land use change and, to a lesser degree, to climatic variables. The lower relation of Fork-tailed Flycatcher density with climatic variables could be due to the lack of significant variations on these variables on the study area included on the wide distribution range of this species (Fraga 2016). Goijman et al. (2015) found high occupancy rates of the Fork-tailed Flycatcher in east central Argentina. White-browed Blackbird was associated with variation in temperature and rainfall (at the regional level and in two sub-regions: agricultural-rangeland and agricultural), showing an association with more rainy and cooler areas. Recent studies in Europe and North America have shown that bird declines are strongly associated to temperature and rainfall (Newton 1998, Morrison and Bolger 2002, Dugger et al. 2004, Szep et al. 2006, Studds and Marra 2007, 2011, Wilson et al. 2011). In particular, species with a low thermal maximum showed the sharpest declines (Jiguet et al. 2010). In this study, climatic variables did not show a temporal trend or significant differences between sub-regions. Additionally, we did not found a relation between variations in density of the Whitebrowed Blackbird with more rainy periods (e.g., in 2005 and 2010), which likely reflect a time lag in the functional response of birds. However, the density of the White-browed Blackbird thrived in wet and temperate habitats, in coincidence with the reported preference of this species for lush wet meadows and humid grasslands (Fraga 2016).
Temporal changes in bird densities in agricultural landscapes have been related to temporal land cover changes in Europe and North America (Chamberlain et al. 2000, Donald et al. 2001, Murphy 2003, Reif et al. 2008, Gaston 2010). In Argentina, previous studies also have shown changes in bird abundance in relation to land use and cover types (Filloy and Bellocq 2007, Gavier-Pizarro et al. 2012, Weyland et al. 2014). A general pattern observed in this study was a gradient from landscapes with scarce forest cover but with more fallow and weedy fields sustaining more Fork-tailed Flycatcher densities, although statistically significant only in the agricultural sub-region, to landscapes with more perennial pastures and annual crops and less fallow and weedy fields and forest cover where the White-browed Blackbird was most abundant.
The density of the Fork-tailed Flycatcher was positively associated to increasing fallow and weedy fields cover and decreasing forst cover. Although this species is reported as a habitat generalist (Mobley and Garcia 2016), in this study it was strongly associated to higher fallow fields cover, which on average occupies a small proportion of studied landscapes. Contrary to this general pattern, in the agricultural-rangeland sub-region this species was positively associated to the first component (this axis went from landscapes dominated by fallow and weedy fields and forest cover, to landscapes dominated by annual crops). Thus, this relationship contradicts its usual association to fallow and weedy fields cover. We believe that in this sub-region the species is mainly avoiding forest cover, even though these ends of the component are also represented by fallow and weedy fields cover. In addition, in this sub-region its response to the fourth component, although explaining only approximately 30% of the variation of the first one, was more strongly associated. Thus, considering both components 1 and 4, the species tends to avoid landscapes with forest cover.
In a shorter time span, Gavier-Pizarro et al. (2012) found a positive relationship between the Fork-tailed Flycatcher and non-plowed fields, indicating a preference for semi-natural habitats, and Filloy and Bellocq (2007) found a negative association with increasing percentage of arable farmland. Additionally, this species prefers nesting in open savannas over closed forest habitats in the Brazilian Cerrado (Marini et al. 2009). Feichtinger and Veech (2013), in a study with Scissor-tailed Flycatcher (Tyrannus forficatus), found a positive association with open land cover types (such as grassland, pasture-hayfield and cropland) and a negative association with forest and scrubland cover. Thus, the maintenance of fallow fields cover in landscapes with low forest cover is probably important for the conservation of birds associated to grassland and savannas. In contrast to the Whitebrowed Blackbird, the general lack of response of Fork-tailed Flycatcher density to climatic factors could be explained by their ecological requirements, which were not influenced by small yearly or spatial variations in the study region.
The White-browed Blackbird, a bird species preferring pastures, agricultural lands and grassland habitats (Narosky and Yzurieta 1987, Stotz et al. 1996, Camperi et al. 2004, Azpiroz et al. 2012, Fraga 2016), showed a relatively consistent response to landscape changes in the agricultural-rangeland and the agricultural sub-regions, increasing in landscapes with more perennial pastures, fallow and weedy fields and annual pastures, and avoiding sites with more forest cover. Its abundance has also been negatively related to increasing native forest in another study conducted in the central part of Argentina for the 2003-2006 period (Schrag et al. 2009). These results probably reflect the species’ reported ability to use a variety of open habitats (Narosky and Yzurieta 1987, Stotz et al. 1996, Camperi et al. 2004, Fraga 2016) as well as its plasticity to changing environments, allowing them to survive in landscapes dominated by open, non-woody habitats and to tolerate the intensive agriculture that typifies the study region. Goijman et al. (2015) found high variability in occupancy estimation over time of the White-browed Blackbird, but it appears to be declining.
Given the high relationship of these two bird species on semi-natural open (non-woody) habitats, such as fallow and weedy fields and annual pastures, we can infer that the conservation of both species would depend on the conservation of these habitat types. Additionally, these remnants of natural or semi-natural habitats would help to maintain a relatively high proportion of avian diversity. Finally, as the studied species have a relatively wide tolerance to habitat variation, further studies would have to be dedicated to grassland dependent species as well as to variables related to the whole bird community, in order to establish more precise and systematic baselines for bird management and conservation at regional scales.
ACKNOWLEDGEMENTS
Funding for this research was provided by INTA projects PNNAT 1128052 and PNNAT 1128053. Part of the funding for avian field surveys was provided by the Canadian Wildlife Service, the US Fish and Wildlife Service (Grant #98210-1-G958), the Western Hemisphere International Office (1997-2002) and the Neotropical Migratory Bird Conservation Act (2003-2005) (Grant #2534). We thank all the people that participated in the field surveys and V. Alessio who did the data entry. P. Marchese provided valuable help to improve the English writing.
LITERATURE CITED
1. AKAIKE H (1974) A new look at the statistical model identification. IEEE Transactions on Automatic Control 19:716-723 [ Links ]
2. ANDERSON DR, BURNHAM KP AND THOMPSON WL (2000) Null hypothesis testing: problems, prevalence, and an alternative. Journal of Wildlife Management 64:912-923 [ Links ]
3. ANDRÉN H, DELIN A AND SEILER A (1997) Population response to landscape changes depends on specialization to different landscape elements. Oikos 80:193-196 [ Links ]
4. ANGERT AL (2009) The niche, limits to species distributions, and spatiotemporal variation in demography across the elevation ranges of two monkey flowers. Proceedings of the National Academy of Sciences 106:19693-19698 [ Links ]
5. AZPIROZ AB, ISACCH JP, DIAS RA, DI GIACOMO AS, FONTANA CS AND PALAREA CM (2012) Ecology and conservation of grassland birds in southeastern South America: a review. Journal of Field Ornithology 83:217-246 [ Links ]
6. BALDI G AND PARUELO JM (2008) Land-use and land cover dynamics in South American temperate grasslands. Ecology and Society 13(2):art6 [ Links ]
7. BARKOWSKA M, PINOWSKI J AND PINOWSKA B (2003) The effect of trends in ambient temperature on egg volume in the Tree Sparrow Passer montanus. Acta Ornithologica 38:5-13 [ Links ]
8. BENNET A, RADFORD JQ AND HASLEM A (2006) Properties of land mosaics: implications for nature conservation in agricultural environments. Biological Conservation 133:250-264 [ Links ]
9. BENTON TG, VICKERY JA AND WILSON JD (2003) Farmland biodiversity: is habitat heterogeneity the key? Trends in Ecology and Evolution 18:182-188 [ Links ]
10. BIBBY CJ, BURGESS ND, HILL DA AND MUSTOE SH (2000) Bird census techniques. Academic Press, London [ Links ]
11. BOLETTA PE, RAVELO AC, PLANCHUELO AM AND GRILLI M (2006) Assessing deforestation in the Argentine Chaco. Forest Ecology and Management 228:108-114 [ Links ]
12. BOTH C AND TE MARVELDE L (2007) Climate change and timing of avian breeding and migration throughout Europe. Climate Research 35:93-105 [ Links ]
13. BOTH C, VAN TURNHOUT CAM, BIJLSMA RG, SIEPEL H, VAN STRIEN AJ AND FOPPEN RPB (2010) Avian population consequences of climate change are most severe for long-distance migrants in seasonal habitats. Proceedings of the Royal Society B 277:1259-1266 [ Links ]
14. BOULINIER T, NICHOLS JD, HINES JE, SAUER JR, FLATHER CH AND POLLOCK KH (2001) Forest fragmentation and bird community dynamics: inference at regional scales. Ecology 82:1159-1169 [ Links ]
15. BOULINIER T, NICHOLS JD, SAUER JR, HINES JE AND POLLOCK KH (1998) Estimating species richness: the importance of heterogeneity in species detectability. Ecology 79:1018-1028 [ Links ]
16. BRADSHAW CJA, BROOK BW, DELEAN S, FORDHAM DA, HERRANDO-PÉREZ S, CASSEY P, EARLY R, SEKERCIOGLU CH AND ARAÚJO MB (2014) Predictors of contraction and expansion of area of occupancy for British birds. Proceedings of the Royal Society B 281:art20140744 [ Links ]
17. BREGMAN TP, SEKERCIOGLU CH AND TOBIAS JA (2014) Global patterns and predictors of bird species responses to forest fragmentation: implications for ecosystem function and conservation. Biological Conservation 169:372-383 [ Links ]
18. BRESCIA V, LEMA D AND PARELLADA G (1998) El fenómeno ENSO y la agricultura pampeana: impactos económicos en trigo, maíz, girasol y soja. Instituto de Economía y Sociología, INTA, Buenos Aires [ Links ]
19. BROWN JH AND LOMOLINO MV (1998) Biogeography. Second edition. Sinauer, Sunderland [ Links ]
20. BUCKLAND ST, ANDERSON DR, BURHAM KP, LAAKE JL, BORCHERS DL AND THOMAS L (2001) Introduction to distance sampling. Estimating abundance of biological populations. Oxford University Press, Oxford [ Links ]
21. BURNHAM KP AND ANDERSON DR (1998) Model selection and inference: a practical information-theoretical approach. Springer-Verlag, New York [ Links ]
22. BURNHAM KP AND ANDERSON DR (2001) Kullback- Leibler information as a basis for strong inference in ecological studies. Wildlife Research 28:111-119 [ Links ]
23. BURNHAM KP AND ANDERSON DR (2002) Model selection and multimodel inference: a practical informationtheoretical approach. Second edition. Springer-Verlag, New York [ Links ]
24. CABRERA AL (1971) Fitogeografía de la República Argentina. Boletín de la Sociedad Argentina de Botánica 14:1-42 [ Links ]
25. CABRERA AL (1976) Regiones fitogeográficas argentinas. Pp. 1-85 en: Enciclopedia argentina de agricultura y jardinería. Tomo 2. Fascículo 1. ACME, Buenos Aires [ Links ]
26. CAMPERI AR, FERRETTI V, CICCHINO AC, SOAVE GE AND DARRIEU AA (2004) Diet composition of the whitebrowed blackbird (Sturnella superciliaris) at Buenos Aires province, Argentina. Ornitología Neotropical 15:299-306 [ Links ]
27. CANAVELLI SB, ZACCAGNINI ME, RIVERA-MILAN FF AND CALAMARI NC (2003) Bird population monitoring as a component of pesticide risk assessment in Argentine agroecosystem. Pp. 1-5 in: Programme and abstracts. Third International Wildlife Management Congress incorporating the 16th Australasian Wildlife Management Society Conference, 1-5 December 2003, Christchurch, New Zealand. Australasian Wildlife Management Society, Christchurch [ Links ]
28. CANAVELLI SB, ZACCAGNINI ME, TORRESIN J, CALAMARI NC, DUCOMMUN MP AND CAPLLONCH P (2004) Monitoreo extensivo de aves en el centro-sur de Entre Ríos. Pp. 349-362 in: ACEÑOLAZA FG (ed) Temas de la biodiversidad del litoral fluvial argentino. Instituto Superior de Correlación Geológica, San Miguel de Tucumán [ Links ]
29. CEREZO A, CONDE MC AND POGGIO SL (2011) Pasture area and landscape heterogeneity are key determinants of bird diversity in intensively managed farmland. Biodiversity and Conservation 20:2649-2667 [ Links ]
30. CHAMBERLAIN DE, FULLER RJ, BUNCE RGH, DUCKWORTH JC AND SHRUBB M (2000) Changes in the abundance of farmland birds in relation to the timing of agricultural intensification in England and Wales. Journal of Applied Ecology 37:771-788 [ Links ]
31. CODESIDO M, GONZÁLEZ FISCHER C AND BILENCA D (2008) Asociaciones entre diferentes patrones de uso de la tierra y ensambles de aves en agroecosistemas de la región pampeana, Argentina. Ornitología Neotropical 19:575-585 [ Links ]
32. CONCEPCIÓN ED AND DÍAZ M (2010) Relative effects of field and landscape-scale intensification on farmland bird diversity in Mediterranean dry cereal croplands. Aspects of Applied Biology 100:245-252 [ Links ]
33. CUETO VR, LOPEZ DE CASENAVE J AND MARONE L (2008) Neotropical austral migrant landbirds: population trends and habitat use in the central Monte desert, Argentina. Condor 110:70-79 [ Links ]
34. DE JUANA E AND GARCÍA AM (2005) Fluctuaciones relacionadas con la precipitación en la riqueza y abundancia de aves de medios esteparios mediterráneos. Ardeola 52:53-66 [ Links ]
35. DONALD PF, GREEN RE AND HEATH MF (2001) Agricultural intensification and collapse of Europe’s farmland bird population. Proceedings of the Royal Society B 268:25-29
36. DONALD PF, SANDERSON FJ, BURFIELD IJ AND VAN BOMMEL FPJ (2006) Further evidence of continentwide impacts of agricultural intensification on European farmland birds, 1990-2000. Agriculture, Ecosystems and Environment 116:189-196 [ Links ]
37. DORMANN CF, ELITH J, BACHER S, BUCHMANN C, CARL G, CARRÉ G, GARCÍA MARQUÉZ JR, GRUBER B, LAFOURCADE B, LEITAO PJ, MÜNKEMÜLLER T, MCCLEAN C, OSBORNE PE, REINEKING B, SCHRÖDER B, SKIDMORE AK, ZURELL D AND LAUTENBACH S (2013) Collinearity: a review of methods to deal with and a simulation study evaluating their performance. Ecography 36:27-46 [ Links ]
38. DUGGER KM, FAABORG J, ARENDT WJ AND HOBSON KA (2004) Understanding survival and abundance of overwintering warblers: does rainfall matter? Condor 106:744-760 [ Links ]
39. DUNN P (2004) Breeding dates and reproductive performance. Birds Climate Change 35:69-87 [ Links ]
40. FEICHTINGER EE AND VEECH JA (2013) Association of Scissor-tailed Flycatchers (Tyrannus forficatus) with specific land-cover types in South-Central Texas. Wilson Journal of Ornithology 125:314-321 [ Links ]
41. FERNÁNDEZ GJ, POSSE G, FERRETTI V AND GABELLI FM (2003) Bird-habitat relationship for the declining Pampas meadowlark populations in the southern Pampas grasslands. Biological Conservation 115:139-148 [ Links ]
42. FEWSTER RM, BUCKAND ST, SIRIWARDENA GM, BAILLIE SR AND WILSON JD (2000) Analysis of population trends for farmland birds using generalized additive models. Ecology 81:1970-1984 [ Links ]
43. FILLOY J AND BELLOCQ MI (2007) Patterns of bird abundance along the agricultural gradient of the Pampean region. Agriculture, Ecosystems and Environment 120:291-298 [ Links ]
44. FIRBANK LG, PETIT S, SMART S, BLAIN A AND FULLER RJ (2008) Assessing the impacts of agricultural intensification on biodiversity: a British perspective. Philosophical Transactions of the Royal Society B 363:777-787 [ Links ]
45. FISCHER J, LINDENMAYER DB AND MANNING AD (2006) Biodiversity, ecosystem function, and resilience: ten guiding principles for commodity production landscapes. Frontiers in Ecology and the Environment 4:80-86 [ Links ]
46. FRAGA RM (2016) White-browed Blackbird (Leistes superciliaris). In: DEL HOYO J, ELLIOTT, A, SARGATAL J, CHRISTIE DA AND DE JUANA E (eds) Handbook of the birds of the world alive. Lynx Edicions, Barcelona (URL: http://www.hbw.com/node/62325) [ Links ]
47. GAO X, HUETE AR, NI W AND MIURA T (2000) Opticalbiophysical relationships of vegetation spectra without background contamination. Remote Sensing of Environment 74:609-620 [ Links ]
48. GASPARRI NI AND GRAU HR (2009) Deforestation and fragmentation of Chaco dry forest in NW Argentina (1972-2007). Forest Ecology and Management 258:913-921 [ Links ]
49. GASTON KJ (2010) Valuing common species. Science 327:154-155 [ Links ]
50. GAVIER-PIZARRO GI, CALAMARI NC, THOMPSON JJ, CANAVELLI SB, SOLARI LM, DECARRE J, GOIJMAN AP, SUAREZ RP, BERNARDOS JN AND ZACCAGNINI ME (2012) Expansion and intensification of row crop agriculture in the Pampas and Espinal of Argentina can reduce ecosystem service provision by changing avian density. Agriculture, Ecosystems and Environment 154:44-55 [ Links ]
51. GOIJMAN AP, CONROY MJ, BERNARDOS JN AND ZACCAGNINI ME (2015) Multi-season regional analysis of multi-species occupancy: implications for bird conservation in agricultural lands in east-central Argentina. PLoS One 10:e0130874 [ Links ]
52. GRAHAM MH (2003) Confronting multicollinearity in ecological multiple regression. Ecology 84:2809-2815 [ Links ]
53. GRAU HR, AIDE TM AND GASPARRI NI (2005) Globalization and soybean expansion into semiarid ecosystems of Argentina. Ambio 34:265-266 [ Links ]
54. GUERRERO I, MORALES MB, OÑATE JJ, GEIGER F, BERENDSE F, DE SNOO G, EGGERS S, PÄRT T, BENGTSSON J, CLEMENT LW, WEISSER WW, OLSZEWSKI A, CERYNGIER P, HAWRO V, LIIRA J, AAVIK T, FISCHER C, FLOHRE A, CARSTEN T AND TSCHARNTKE T (2012) Response of ground-nesting farmland birds to agricultural intensification across Europe: landscape and field level management factors. Biological Conservation 152:74-80 [ Links ]
55. HEIKKINEN RK, LUOTO M, VIRKKALA R AND RAINIO K (2004) Effects of habitat cover, landscape structure and spatial variables on the abundance of birds in an agricultural forest mosaic. Journal of Applied Ecology 41:824-835 [ Links ]
56. HOLT RD (2003) On the evolutionary ecology of species ranges. Evolutionary Ecology Research 5:159-178 [ Links ]
57. HUETE AR, MIURA DK, RODRIGUEZ EP, GAO X AND FERREIRA LG (2002) Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sensing of Environment 83:195-213 [ Links ]
58. INDEC (1995) Encuesta nacional agropecuaria 93. Instituto Nacional de Estadística y Censos, Buenos Aires [ Links ]
59. JAHN AE, LEVEY DJ, CUETO VR, LEDEZMA JP, TUERO DT, FOX JW AND MASSON D (2013) Long-distance bird migration within South America revealed by lightlevel geolocators. Auk 130:223-229 [ Links ]
60. JIANG Z, HUETE AR, DIDAN K AND MIURA T (2008) Development of a two-band Enhanced Vegetation Index without a blue band. Remote Sensing of Environment 112:3833-3845 [ Links ]
61. JIGUET F, DEVICTOR V, OTTVALL R, VAN TURNHOUT C, VAN DER JEUGD H AND LINDSTRÖM A (2010) Bird population trends are linearly affected by climate change along species thermal ranges. Proceedings of the Royal Society B 277:3601-3608 [ Links ]
62. JOHNSON JB AND OMLAND KS (2004) Model selection in ecology and evolution. Trends in Ecology and Evolution 192:101-108 [ Links ]
63. JOSEPH L (1997) Towards a broader view of Neotropical migrants: consequences of a re-examination of austral migration. Ornitología Neotropical 8:31-36 [ Links ]
64. KARSH MB AND MCIVER DC (2009) Impacts of climate extremes on biodiversity in the Americas. Adaptation and Impacts Research Division, Environment Canada, Toronto [ Links ]
65. LEMOINE N, BAUER HG, PEINTINGER M AND BÖHNINGGAESE K (2007) Effects of climate and land-use change on species abundance in a central European bird community. Conservation Biology 21:495-503 [ Links ]
66. LEWIS JP, NOETINGER S, PRADO DE AND BARBERIS IM (2009) Woody vegetation structure and composition of the last relicts of Espinal vegetation in subtropical Argentina. Biodiversity and Conservation 18:3615-3628 [ Links ]
67. MACCHI L, GRAU HR, ZELAYA PV AND MARINARO S (2013) Trade-offs between land use intensity and avian biodiversity in the dry Chaco of Argentina: a tale of two gradients. Agriculture, Ecosystems and Environment 174:11-20 [ Links ]
68. MAGURRAN AE, BAILLIE SR, BUCKLAND ST, MCP DICK J, ELSTON DA, SCOTT EM, ROGNVALD IS, SOMERFIELD PJ AND WATT AD (2010) Long-term datasets in biodiversity research and monitoring: assessing change in ecological communities through time. Trends in Ecology and Evolution 25:574-582 [ Links ]
69. MARINI MÂ, LOBO Y, LOPES LE, FRANÇA LF AND PAIVA LV (2009) Breeding biology of Tyrannus savana (Aves, Tyrannidae) in Cerrado of central Brazil. Biota Neotropica 9:55-63 [ Links ]
70. MATSUSHITA B, YANG W, CHEN J, ONDA Y AND QIU G (2007) Sensitivity of the Enhanced Vegetation Index (EVI) and Normalized Difference Vegetation Index (NDVI) to topographic effects: a case study in high-density cypress forest. Sensors 7:2636-2651 [ Links ]
71. MATTEUCCI S (2012a) Ecorregión Espinal. Pp. 349-390 in: MORELLO J, MATTEUCCI S, RODRÍGUEZ AF AND SILVA ME (eds) Ecorregiones y complejos ecosistémicos argentinos. Orientación Gráfica Editora, Buenos Aires [ Links ]
72. MATTEUCCI S (2012b) Ecorregión Pampa. Pp. 391-445 in: MORELLO J, MATTEUCCI S, RODRÍGUEZ AF AND SILVA ME (eds) Ecorregiones y complejos ecosistémicos argentinos. Orientación Gráfica Editora, Buenos Aires [ Links ]
73. MCCUNE B, GRACE JB AND URBAN DL (2002) Analysis of ecological communities. MjM Software Design, Gleneden Beach [ Links ]
74. MESSINA C, HANSEN J AND HALL AJ (1999) Land allocation conditioned on El Niño Southern Oscillation phases in the Pampas of Argentina. Agricultural Systems 20:1-16 [ Links ]
75. MOBLEY J AND GARCIA EFJ (2016) Fork-tailed Flycatcher (Tyrannus savana). In: DEL HOYO J, ELLIOTT, A, SARGATAL J, CHRISTIE DA AND DE JUANA E (eds) Handbook of the birds of the world alive. Lynx Edicions, Barcelona (URL: http://www.hbw.com/node/57488) [ Links ]
76. MORRISON SA AND BOLGER DT (2002) Variation in a sparrow's reproductive success with rainfall: food and predator-mediated processes. Oecologia 133:315-324 [ Links ]
77. MURPHY MT (2003) Avian population trends within the evolving agricultural landscape of eastern and central United States. Auk 120:20-34 [ Links ]
78. NAROSKY T AND YZURIETA D (1987) Guía para la identificación de las aves de Argentina y Uruguay. Asociación Ornitológica del Plata and Vázquez Mazzini Editores, Buenos Aires [ Links ]
79. NEWBOLD T, SCHARLEMANN JPW, BUTCHART SHM, SEKERCIOGLU CH, ALKEMADE R, BOOTH H AND PURVES DW (2013) Ecological traits affect the response of tropical forest bird species to land-use intensity. Proceedings of the Royal Society B 280:2012-2131 [ Links ]
80. NEWTON I (1998) Population limitation in birds. Academic Press, London [ Links ]
81. NIVEN DK, BUTCHER GS, BANCROFT GT, MONAHAN WB AND LANGHAM G (2009) Birds and climate change. Ecological disruption in motion. A briefing for policymakers and concerned citizens on Audubon’s analyses of North American bird movements in the face of global warming. Audubon, New York
82. NORI J, LESCANO JN, ILLOLDI-RANGEL P, CABRERA MR AND LEYNAUD GC (2012) The conflict between agricultural expansion and priority conservation areas: making the right decisions before it’s too late. Biological Conservation 159:507-513
83. OESTERHELD M (2008) Impacto de la agricultura sobre los ecosistemas. Fundamentos ecológicos y problemas más relevantes. Ecología Austral 18:337-346 [ Links ]
84. PARUELO JM, GUERSCHMAN JP AND VERON S (2005) Expansión agrícola y cambios en el uso del suelo. Ciencia Hoy 15:14-23 [ Links ]
85. PINHEIRO JC AND BATES DM (2000) Mixed-effects models in s and s-plus. Springer-Verlag, New York [ Links ]
86. PINHEIRO JC, BATES D, DEBROY S, SARKAR D AND R CORE TEAM (2013) nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-113. R Foundation for Statistical Computing, Vienna (URL: https://cran.rproject.org/package=nlme) [ Links ]
87. REIF J, VORÍŠEK P, ŠTASTNÝ K, BEJCEK V AND PETR J (2008) Agricultural intensification and farmland birds: new insights from a central European country. Ibis 150:596-605
88. SARKAR D (2008) Lattice: multivariate data visualization with R. Springer, New York [ Links ]
89. SCHRAG AM, ZACCAGNINI ME, CALAMARI NC AND CANAVELLI SB (2009) Climate and land-use influences on avifauna in central Argentina: broad-scale patterns and implications of agricultural conversion for biodiversity. Agriculture, Ecosystems and Environment 132:135-142 [ Links ]
90. SIRIWARDENA GM, BAILLIE SR, BUCKLAND ST, FEWSTER RM, MARCHANT JH AND WILSON JD (1998) Trends in the abundance of farmland birds: a quantitative comparison of smoothed Common Birds Census indices. Journal of Applied Ecology 35:24-43 [ Links ]
91. SORIANO A, LEÓN RJC, SALA OE, LAVADO RS, DEREGIBUS VA, CAUHÉPÉ MA, SCAGLIA OA, VELÁZQUEZ CA Y LEMCOFF JH (1991) Río de la Plata grasslands. Pp. 367-407 en: COUPLAND RT (ed) Ecosystems of the world. Volume 8A. Natural grasslands: introduction and Western Hemisphere. Elsevier, Amsterdam [ Links ]
92. STOTZ DF, FITZPATRICK FW, PARKER TA AND MOSKOVITS DK (1996) Neotropical birds. Ecology and conservation. University of Chicago Press, Chicago [ Links ]
93. STUDDS CE AND MARRA PP (2007) Linking fluctuations in rainfall to nonbreeding season performance in a long-distance migratory bird, Setophaga ruticilla. Climate Research 35:115-122 [ Links ]
94. STUDDS CE AND MARRA PP (2011) Rainfall induced changes in food availability modify the spring departure programme of a migratory bird. Proceedings of the Royal Society B 278:3437-3443 [ Links ]
95. SZEP TA, MØLLER AP, PIPER S, NUTTALL R, SZABÓ ZD AND PAP PL (2006) Searching for potential wintering and migration areas of a Danish Barn Swallow population in South Africa by correlating NDVI with survival estimates. Journal of Ornithology 147:245-253 [ Links ]
96. THOMAS L, BUCKLAND ST, BURNHAM KP, ANDERSON DR, LAAKE JL, BORCHERS DL AND STRINDBERG S (2002) Distance sampling. Pp. 544-552 in: EL-SHAARAWI AH AND PIEGORSCH WW (eds) Encyclopedia of environmetrics. John Wiley and Sons, Chichester [ Links ]
97. VICKERY JA, FEBER RE AND FULLER RJ (2009) Arable field margins managed for biodiversity conservation: a review of food resource provision for farmland birds. Agriculture, Ecosystems and Environment 133:1-13 [ Links ]
98. VIGLIZZO EF, PORDOMINGO AJ, CASTRO MG, LÉRTORA FA AND BERNARDOS JN (2004) Scale dependent controls on ecological functions in agroecosystems of Argentina. Agriculture, Ecosystems and Environment 101:39-51 [ Links ]
99. VIGLIZZO EF, ROBERTO ZE, LÉRTORA FA, LÓPEZ GAY E AND BERNARDOS JN (1997) Climate and land-use change in field-crop ecosystems of Argentina. Agriculture, Ecosystems and Environment 66:61-70 [ Links ]
100. WEYLAND F, BAUDRY J Y GHERSA CM (2014) Rolling Pampas agroecosystem: which landscape attributes are relevant for determining bird distributions? Revista Chilena de Historia Natural 87:art1 [ Links ]
101. WILSON S, LADEAU SL, TØTTRUP AP AND MARRA PP (2011) Range-wide effects of breeding- and nonbreeding-season climate on the abundance of a Neotropical migrant songbird. Ecology 92:1789-1798 [ Links ]
102. ZACCAGNINI ME AND CALAMARI NC (2001) Labranzas conservacionistas, siembra directa y biodiversidad. Pp. 29-68 in: PANIGATTI JL, BUSCHIAZZO D AND MARELLI H (eds) Siembra directa II. INTA, Buenos Aires [ Links ]
103. ZACCAGNINI ME, CANAVELLI SB, CALAMARI NC AND SCHRAG AM (2010) Regional bird monitoring as a tool for predicting the effects of land use and climate change on Pampas biodiversity. Pp. 39-52 in: DALLMEIER F, FENECH A, MACIVER D AND SZARO R (eds) Climate change, biodiversity and sustainability in the Americas: impacts and adaptations. Smithsonian Institution and Rowman & Littlefield, Washington DC [ Links ]
104. ZAK MR, CABIDO M, CÁCERES D AND DÍAZ S (2008) What drives accelerated land cover change in central Argentina? Synergistic consequences of climatic, socioeconomic and technological factors. Environmental Management 42:181-189 [ Links ]














