Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos argentinos de pediatría
versión impresa ISSN 0325-0075
Arch. argent. pediatr. vol.111 no.1 Buenos Aires ene./feb. 2013
ORIGINAL ARTICLE
Sedation and analgesia in children undergoing invasive procedures
Loreto Godoy M.a, M.D., Paola Pino A.a, Registered Nurse, Gulliana Córdova L.a, M.D., Juan Andrés Carrasco O.a, M.D. and Andrés Castillo M.a, M.D.
a. Department of Pediatrics. Hospital Clínico. Pontificia Universidad Católica de Chile.
E-mail: Andrés Castillo M., M.D. acastill@med.puc.cl
Funded by: ILSI Argentina.
Conflict of interest: None.
Received: 02-07-2012
Accepted: 09-25-2012
http://dx.doi.org/10.5546/aap.2013.22
ABSTRACT
Introduction. As a result of the increased number of both diagnostic and therapeutic procedures in pediatric outpatients, sedation and analgesia have gained relevance in this context.
Objective. To characterize the type of sedation and analgesia used by pediatric sedation teams in procedures done outside the operating room, as well as its safety and outcome.
Population and Methods. All procedures performed in 1 month to 5 year old patients under intravenous sedation with midazolam, ketamine, propofol or lidocaine were analyzed over a 14-month period. The Ramsay sedation scale and the CHEOPS pain scale were used to determine the response to the sedation and analgesia administered.
Results. A total of 186 procedures were analyzed. The results of the evaluation of response to sedation and analgesia indicated that an adequate deep sedation was obtained in 98% of cases, and that an adequate analgesia was achieved in 92% of patients. Around 12% of the procedures were associated with adverse events, all related to the airways, and none was serious. The only statistically significant endpoint associated with adverse events were procedures which involved airway interventions, i.e., fibrobronchoscopy, upper gastrointestinal endoscopy or transesophageal echocardiogram, with an OR of 6.27 (95% CI: 1.28-30.63; p = 0.023).
Conclusions. In this group of patients, intravenous outpatient sedation and analgesia administered by a specialized team were safe and effective.
Key words: Conscious sedation; Deep sedation; Analgesia; Pediatrics.
INTRODUCTION
Infants and toddlers are not able to understand most of the situations involved in the treatment and diagnosis of their condition. Even older children, who are able to understand the procedure to be performed on them, are anxious and this makes them fight against the procedure, especially if it is painful. Such lack of cooperation makes any procedure in pediatric patients difficult, both for the patient and the medical staff, especially if pain is involved. At the same time, it is important that when the procedure is over the patient forgets this traumatic experience. For this reason, any pediatric procedure should provide a very adequate control of pain and anxiety.1
On the other hand, given the growing complexity of pediatrics, there is an increasing trend towards providing an efficient and cost-effective care to all pediatric patients. For this reason, diagnostic or therapeutic procedures in pediatric outpatients are becoming more and more frequent.2 In this context, safe sedation and analgesia are particularly relevant.3
The objective of this study is to characterize the type of sedation and analgesia used by a team trained in pediatric sedation, in children undergoing different diagnostic and therapeutic procedures outside the operating room, as well as the efficacy and safety of the drugs used.
PATIENTS AND METHODS
A prospective study was conducted on a series of cases managed in the Pediatric Department of the Clinical Hospital at the Pontificia Universidad Católica de Chile, at a ward specially authorized to carry out these procedures. The sample corresponds to all procedures performed under intravenous sedation between May 2010 and July 2011. Patients undergoing such procedures had to meet the following inclusion criteria:
. Age from 1 month to 5 years and 11 months old.
. Classification according to the American Society of Anesthesiologists (ASA) criteria:
ASA I: healthy patient, or ASA II: patient with mild systemic disease, well controlled and not disabling.4
. Fasting according to ASA: clear fluids for 2 hours, breast milk for 4 hours, other food for 6 hours.4
The exclusion criteria included:
. Receiving a sedation schedule different from: midazolam plus ketamine, propofol plus lidocaine, or midazolam plus propofol plus lidocaine.
Procedural sedation and analgesia was managed by a pediatric sedation team, made up of a doctor specialized in pediatric intensive care, a pediatric intensive care nurse, and a senior nurse technician. The medical team consisted of four critical care physicians or intensivists, each one in charge of the procedures for one week at a time. Drugs prescribed and their dosage were selected at each physician's discretion.
For this study, a sedation record sheet was made up and completed before the procedure, together with the informed consent.
During the procedure, the following data were recorded: vital signs, oxygen requirement, administered drugs, associated adverse events, and measures taken to solve them. An adverse event was defined as any event resulting from sedation and analgesia that may harm the patient, such as hypotension, bradycardia, stridor or hypoxemia (defined as oxygen saturation < 85% by pulse oxymeter). All patients received supplemental oxygen with a nasal cannula during sedation (1- 2 liters/minute). Once the procedure was over, vital signs and the recovery scale score were recorded every 10 minutes.
In order to determine the response to sedation and analgesia, two scales were used:
. Ramsay sedation scale:5 evaluation of deep sedation: 1: patient awake, 6: patient asleep with no response to any stimuli (Table 1).
. CHEOPS pain scale:6 use of clinical parameters to determine a minimum score of 4 (no pain at all) and a maximum score of 13 (severe pain) (Table 2).
TABLE 1. Ramsay scale

Adequate moderate sedation: Ramsay 3-4.
Adequate deep sedation: Ramsay 5-6.
ASA: American Society of Anesthesiologists.
TABLE 2. CHEOPS scale

For this study, adequate analgesia was defined as a score of <7.
For the purpose of this study, adequate moderate sedation was defined as a Ramsay score of 3 or 4, adequate deep sedation, as a Ramsay score of 5 or 6; and adequate analgesia was considered a CHEOPS score < 7. The CHEOPS scale does not have an unequivocal cut-off point; however, a score of < 7 has been used in different studies to define the absence of pain.7-12
For the decision to discharge the patient from the procedure ward, the objective discharge criteria of the modified Aldrete score were used,8 which assesses different clinical parameters, with a maximum score of 14: patient with no sedation effect (Table 3).
TABLE 3. Modified Aldrete scale
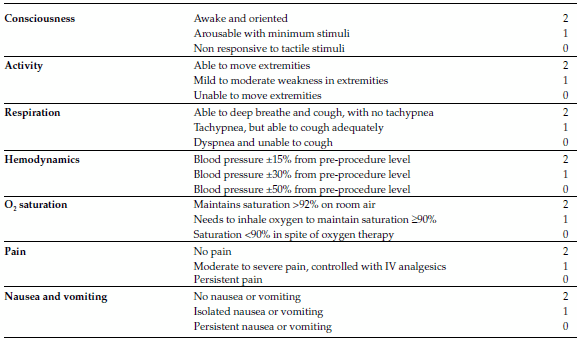
Recovery discharge criteria: a score of =12 and no category with a score of 0.
Statistical Analysis
Categorical endpoints were described in terms of number and percentage, while continuous endpoints were described in terms of median and interquartile range. The frequency of adverse events in categorical endpoints (type of procedure, ASA classification and combination of drugs used) was compared using Fisher's exact test; while continuous endpoints (median age and weight) were compared using Mann-Whitney test. Finally, a logistic regression model was prepared for the presence of adverse events, including the independent endpoints mentioned above. The model results were expressed as adjusted odds ratios, with their corresponding 95% confidence intervals. Any p value < 0.05 was considered statistically significant. Data were analyzed using the SPSS® software.
The study was approved by Ethics Committee of the Clinical Hospital at the Pontificia Universidad Católica de Chile.
RESULTS
During the follow-up period, 215 procedures were performed using intravenous sedation and analgesia in 1 month to 5 years and 11 months old patients. Of these, 29 procedures were excluded because they had been conducted using drug combinations other than midazolam plus ketamine; propofol plus lidocaine; or midazolam plus propofol plus lidocaine. Eventually, 186 procedures were included in the analysis.
Out of these, 58% (n = 107) were performed in females. The median age of patients was 3 years old, ranging from 1 month to 5 years and 11 months old. The median weight was 14 kilos, ranging from 3.4 to 38 kilos.
The most common diagnosis at admission was hematooncological disease (n = 64; 34%), followed by gastrointestinal disease (n = 43; 23%), neurological disease (n = 30; 16%), respiratory disease (n = 28; 15%), and other diagnoses (n = 21; 11%).
A total of 63% (n = 117) of procedures were performed in patients with an ASA II classification, while the rest had an ASA I classification. Of these, 63% (n = 117) required a peripheral line to be placed before the procedure; the rest had a temporary or definitive central line. Fasting before the procedure lasted for a median of 10 hours, ranging from 5 to 17 hours.
The most common procedure was a lumbar puncture with intrathecal chemotherapy or myelography (n = 64; 34%); followed by fibrobronchoscopy, upper gastrointestinal endoscopy or transesophageal echocardiogram (n = 59; 32%); liver, kidney, muscle, skin or thyroid biopsy (n = 26; 14%); percutaneous central venous catheter insertion (n = 9; 5%); single lumbar puncture, ventricular or valve reservoir puncture (n = 6; 3%); and others (n = 22; 12%) (suture removal, surgical wound care, drain removal, etc.).
Procedures lasted for a median of 10 minutes, ranging from 5 to 60 minutes; in general, the shortest were oncological procedures, followed by endoscopies, and biopsies in the last place.
When sedation effectiveness was evaluated, a Ramsay score of =5 (adequate deep sedation) was achieved in 76% (n = 141) of the cases at the beginning of the sedation process, and in 98% (n = 183) of the cases at the end of the sedation process. In relation to analgesia, a CHEOPS score of < 7 (adequate analgesia) was obtained in 44% (n = 82) of the cases at the beginning of the procedure and in 92% (n = 171) at the end of it.
Using the modified Aldrete scale as a discharge criterion, patients were discharged from the procedure ward with a score of 12 or more and with no category with a score of 0.
Recovery lasted for a median of 10 minutes, ranging from 10 to 40 minutes. Most of the patients were transferred with 12 points (n = 183; 98%), the rest had 13 points at their first follow-up visit.
Drugs used included midazolam, propofol and ketamine, all given intravenously. In all cases where intravenous analgesia was not indicated, topical or subcutaneous lidocaine was used. The most commonly used combination was midazolam plus ketamine (n = 128; 69%), followed by propofol plus lidocaine (n = 40; 21%), and midazolam plus propofol plus lidocaine (n = 18; 10%) (Table 4).
TABLE 4. Combinations of drugs used, adverse events and type of procedure

*AW: airway.
**AE: adverse event.
Of all procedures, 12% (n = 22) was associated with adverse events, including hypoxemia (defined as oxygen saturation <85% by pulse oxymeter) (n = 19) and stridor (n = 3).
Table 4 shows the differential analysis of complications related to the sedation and analgesia schedule used.
Hypoxemia episodes were reverted using oxygen, changing airway positioning, aspirating secretions and, in 13 of 19 cases (68%), with positive pressure ventilation; none of the hypoxemia episodes were associated with bradycardia nor required intubation. Stridor was reverted using nebulized racemic adrenaline.
The assessment of endpoints most associated with adverse events showed that age, weight, type of procedure, ASA classification, and combination of drugs used were statistically significant (Tables 5 and 6).
TABLE 5. Univariate analysis. Categorical endpoints

* Group 1: midazolam plus ketamine.
** Group 2: propofol plus lidocaine, or midazolam plus propofol plus lidocaine.
ASA: American Society of Anesthesiologists.
TABLE 6. Univariate analysis. Continuous endpoints
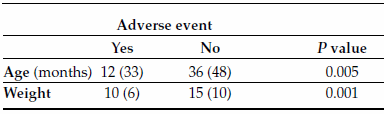
Values around the median (interquartile range).
Patients with adverse events were significantly younger than those with no adverse events (median = 12 months, IQR = 33 months vs. median = 36 months, IQR = 48 months; p = 0.005).
Patients with adverse events weighted significantly less than those with no adverse events (median = 10 kg, IQR = 6 kg vs. median = 15 kg, IQR = 10 kg; p = 0.01).
In terms of the review, procedures were grouped as follows:
. Airway intervention procedures: fibrobronchoscopy, upper gastrointestinal endoscopy or transesophageal echocardiogram.
. Procedures without airway intervention: the rest of the procedures.
Airway intervention procedures caused a more significant number of adverse events than procedures without airway intervention (n = 17; 29% vs. n = 5; 4%; p = 0.001).
Procedures performed in ASA I patients caused a more significant number of adverse events than in ASA II patients (n = 14; 20% vs. n = 8; 7%; p = 0.009).
In terms of the review, the combination of drugs used was grouped as follows:
. Group 1: midazolam plus ketamine.
. Group 2: propofol plus lidocaine, or midazolam plus propofol plus lidocaine.
Procedures performed using combination 2 caused a more significant number of adverse events than combination 1 (n = 14; 24% vs. n = 8; 6%; p = 0.001).
Lastly, the risk of adverse event occurrence was assessed using a multivariate model, which included the following endpoints: weight, age, type of procedure, ASA classification, and drug combination used.
In this analysis, the only endpoint with a statistically significant association with the occurrence of adverse events was the type of procedure, with an OR of 6.27 (95% CI: 1.28-30.63; p = 0.023) for the airway intervention procedures (Table 7).
TABLE 7. Multivariate analysis

* Group 2: propofol plus lidocaine, or midazolam plus propofol plus lidocaine.
ASA: American Society of Anesthesiologists.
DISCUSSION
The increasing number of invasive diagnostic or therapeutic procedures in children has led to the implementation of procedures and teams, generally consisting of pediatric intensive care staff, specialized in outpatient sedation and analgesia. This study evaluated 186 procedures with such characteristics, and assessed the response and safety of sedation and analgesia, as well as the risk factors associated with adverse events.
In relation to the response to sedation and analgesia, our team decided to use the Ramsay and CHEOPS scales, because they are easy to apply and reproduce by different operators.7,9,14 When these two scales were applied to this group of patients, our team observed that most procedures achieved adequate deep sedation and analgesia, according to the specified cut-off points.
In relation to sedation and analgesia safety, adverse events were seen in 22 procedures (12%), and 100% of these were related to airway complications: stridor (n = 3) or hypoxemia episode (n = 19). This situation is similar to that published in other series,15-19 which describe a 3-30% of respiratory complication rate.
The midazolam-ketamine group had a lower incidence of hypoxemia events compared to the other groups; however, it should be noted that it occurred in 3 out of 8 airway intervention procedures, vs. 100% in the rest of the groups.
Stridor occurred in three patients, all undergoing airway intervention procedures, so we believe it is related to the type of procedure.
The risk factors associated with a higher number of adverse events were: younger age and lower weight, drug combinations with propofol and, specially, airway intervention procedures; all of them characteristics previously described in the bibliography.20,21
Initially, the ASA classification was also statistically significant, with a higher risk for ASA I patients, which is considered to be accountable for the fact that most patients undergoing airway intervention procedures were ASA I (ASA I: 57% airway intervention procedures vs. ASA II: 17%).
When adjusted by the remaining endpoints, only airway intervention is statistically significant.
In relation to positive pressure ventilation requirement, in our group a higher number of patients requiring such intervention was observed in comparison to the rest of the mentioned series (13 of 19 patients, 68%),15-19 which may be related to a younger median age and to a higher number of airway intervention procedures. This is related to the fact that in our procedure ward, as per protocol, patients receive positive pressure ventilation if saturation is not achieved rapidly (defined as 10 seconds). It should be noted that a skilled advanced management of pediatric airways is critical for this task. For this reason, it is essential that outpatient sedation is administered by a specially trained team, because the administration of sedation and analgesia and its progress conform a continuum that may lead to severe respiratory depression or cardiovascular collapse. At this point, published guidelines and recommendations gain relevance and they should be complemented and adapted to the actual situation of each facility.22-24
Among the study's weaknesses, we found a relatively small "n" and a significant group heterogeneity, in relation to both the procedure and the sedation and analgesia schedule used, which renders the possibility of performing a global analysis more difficult.
Among the study's strengths, we found a group of patients younger than 6 years old, with a median age of 3 years old, i.e., a reduced age range that allows to assess more clearly adverse events.
Another strength of this study was the fact that the number of procedures with airway compromise was significant in the series (n = 59; 32% of procedures), which resulted in a better evaluation of adverse events in this subgroup.
As far as the conduction of outpatient procedures it is important to consider the significant reduction of procedural costs given that not using the operating room represents a 75% cost reduction to our institution. In fact, costs may be reduced not only because of not using the operating room, but also, as evidenced by Pershad, et al.,25 by decreasing and optimizing costs depending on the drugs used.
CONCLUSION
In this group of patients, intravenous sedation and analgesia administered by a team specialized in outpatient procedures were safe and effective. However, it will be highly relevant to develop prospective protocols to assess the costeffectiveness ratio and the safety of certain drug combinations, so as to establish practical guidelines implemented by physicians specially trained to perform outpatient procedures.
Acknowledgments
To the team of professionals that work at the procedure ward of the Pediatrics Department at the UC Clinical Hospital.
To Jaime Cerda, M.D., specialized in Public Health, Department of Public Health of PUC, who helped with data statistical analysis.
1. Krauss B, Green S. Sedation and analgesia for procedures in children. N Engl J Med 2000;342(13):938-45. [ Links ]
2. Kaplan R, Yang C. Sedation and Analgesia in Pediatric Patients for Procedures Outside the Operating Room. Anesthesiol Clin North America 2002;20(1):181-94. [ Links ]
3. Cravero J, Blike G. Review of Pediatric Sedation. Anesth Analg 2004;99(5)1355-64. [ Links ]
4. Shankar V, Deshpande J. Procedural Sedation in the Pediatric Patient. Anesthesiol Clin North America 2005;23(4):635-54. [ Links ]
5. Ramsay M, Savege T, Simpson B, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J 1974;2(5920):656-9. [ Links ]
6. McGrath P, Johnston G, Goodman J. CHEOPS: a behavioral scale for rating postoperative pain in children. En: Fields HL (ed). Advances in Pain Research and Therapy. Nueva York: Raven Press; 1985.Págs.395-402. [ Links ]
7. Hesselgard K, Larsson S, Romner B, Strömblad L, Reinstrup P. Validity and reliability of the Behavioural Observational Pain Scale for postoperative pain measurement in children 1-7 years of age. Pediatr Crit Care Med 2007;8(2):102-8. [ Links ]
8. Bringuier S, Picot M, Dadure C, Rochette A, et al. A prospective comparison of post-surgical behavioral pain scales in preschoolers highlighting the risk of false evaluations. Pain 2009;145(1-2):60-8. [ Links ]
9. Suraseranivongse S, Santawat U, Kraiprasit K, Petcharatana S, et al. Cross-validation of composite pain scale for preschool children within 24 hours of surgery. Br J Anaesth 2001;87(3):400-5. [ Links ]
10. McCarty E, Mencio G, Walker L, Green N. Ketamine sedation for the reduction of children's fractures in emergency department. J Bone Joint Surg Am 2000;82-A(7):912-8. [ Links ]
11. Suraseranivongse S, Chowvanayotin S, Pirayavaraporn S, Kongsayreepong S, et al. Effect of bupivacaine with epinephrine wound instillation for pain relief after pediatric inguinal herniorrhaphy and hydrocelectomy. Reg Anesth Pain Med 2003;28(1):24-8. [ Links ]
12. Tay C, Tan S. Diclofenac or paracetamol for analgesia in paediatric myringotomy outpatients. Anaesth Intensive Care 2002;30(1):55-9. [ Links ]
13. Aldrete J. The post-anesthesia recovery score revisited. J Clin Anaesth 1995;7(1):89-91. [ Links ]
14. De Jonghe B, Cook D, Appere De Vecchi C, Guyatt G, et al. Using and understanding sedation scoring systems: a systematic review. Intensive Care Med 2000;26(3):275-85. [ Links ]
15. Vardi A, Salem Y, Padeh S, Paret G, Barzilay Z. Is propofol safe for procedural sedation in children? A prospective evaluation of propofol versus ketamine in pediatric critical care. Crit Care Med 2002;30(6):1231-6. [ Links ]
16. Shah A, Mosdossy G, McLeod S, Lehnhardt K, et al. A blinded, randomized controlled trial to evaluate ketaminepropofol versus ketamine alone for procedural sedation in children. Ann Emerg Med 2011;57(5):425-33 e2. [ Links ]
17. Roback M, Wathen J, Bajaj L, Bothner J. Adverse events associated with procedural sedation and analgesia in a pediatric emergency department: a comparison of common parenteral drugs. Acad Emerg Med 2005;12(6):508-13. [ Links ]
18. Peña B, Krauss B. Adverse events of procedural sedation and analgesia in a pediatric emergency department. Ann Emerg Med 1999;34(4 pt1):483-91. [ Links ]
19. Pitetti R, Singh S, Pierce M. Safe and efficacious use of procedural sedation and analgesia by nonanesthesiologists in a pediatric emergency department. Arch Pediatr Adolesc Med 2003;157(11):1090-6. [ Links ]
20. Thakkar K, El-Serag H, Mattek N, Gilger M. Complications of pediatric EGD: a 4-year experience in PEDS-CORI. Gastrointest Endosc 2007;65(2):213-21. [ Links ]
21. Barbi E, Gerarduzzi T, Marchetti F, Neri E, et al. Deep Sedation With Propofol by Nonanesthesiologists: a prospective pediatric experience. Arch Pediatr Adolesc Med 2003;157(11):1097-3. [ Links ]
22. Garcia Roig C, Caprotta G, de Castro M, Germ R, Lagomarsino E. Analgesia y sedación en procedimientos pediátricos. Parte 1: Aspectos generales, escalas de sedación y valoración del dolor. Arch Argent Pediatr 2008;106(5):429-34. [ Links ]
23. Garcia Roig C, Caprotta G, de Castro M, Germ R, Lagomarsino E. Analgesia y sedación en procedimientos pediátricos. Parte 2: Requerimientos y medicación. Arch Argent Pediatr 2008;106(6):524-32. [ Links ]
24. Cravero J, Beach M, Blike G, Gallagher S, et al. The incidence and nature of adverse events during pediatric sedation/anesthesia with propofol for procedures outside the operating room: A report from the Pediatric Sedation Research Consortium. Anesth Analg 2009;108(3):795-804. [ Links ]
25. Pershad J, Todd K, Waters T. Cost-effectiveness analysis of sedation and analgesia regimens during fracture manipulation in the pediatric emergency department. Pediatr Emerg Care 2006;22(10):729-36. [ Links ]














