Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos argentinos de pediatría
versión impresa ISSN 0325-0075versión On-line ISSN 1668-3501
Arch. argent. pediatr. vol.116 no.6 Buenos Aires dic. 2018
http://dx.doi.org/10.5546/aap.2018.e736
ORIGINAL ARTICLE
http://dx.doi.org/10.5546/aap.2018.eng.e736
Is there an association between vitamin D level and iron deficiency in children?
Meriç Kaymak Cihan, M.Da,b and Elif Ünver Korğalı, M.D.c
a. Division of Pediatric Hematology and Oncology, Department of Pediatrics, Cumhuriyet University Faculty of Medicine, Sivas.
b. Department of Pediatric Hematology and Oncology, Dr. Abdurrahman Yuraslan Ankara Oncology Training and Education Hospital, Ankara.
c. Department of Pediatrics, Cumhuriyet University Faculty of Medicine, Sivas. Turkey.
E-mail address: Merig Kaymak Cihan, M.D.: merckaymak@gmail.com
Funding: None.
Conflict of interest: None.
Received: 1-23-2018
Accepted: 8-16-2018
ABSTRACT
Introduction. Vitamin D (VitD) affects the erythropoiesis. The aim of this study was to evaluate the association between maternal/child 25-OH VitD (25-OHD) levels and iron deficiency (ID) and anemia (IDA) in children aged 6 months-5 years.
Population and methods. Between September 2014 and January 2016 children who were admitted to outpatient clinic were included to study. We excluded the children with acute or chronic infection, malnutrition, chronic disease and preterm birth history. Complete blood count, serum iron, total iron binding capacity, ferritin, 25-OHD levels were examined from children and their mothers. Iron and VitD supplementation during infancy and pregnancy and breastfeeding history were questioned.
Results. The study included totally 117 children. There were 67 children with ID/IDA [Group 1, mean age (years):2.05±1.24 (0.5-5)] and 50 normal children [Group 2, mean age (years): 1.87±1.12 (0.58-5)]. There were more VitD deficient children and mothers in Group 1 than in Group 2 (respectively, children 49.3 % vs. 20 % p=0.002; mothers 94 % vs.64 %, p=<0.001). There was a positive correlation between hemoglobin levels of children and maternal/child 25-OHD.The independent risk factors for IDA in children were longer exclusively breastfeeding time (odds ratio [OR], 0.35; 95 % confidence interval [CI], 0.1550.789; p=0.011), shorter duration of regular iron supplementation during infancy and pregnancy (infancy: OR,1.69; 95 % CI 1.148-2.508; p=0.008. pregnancy: OR,1.39; 95 % 0,1.070-1.820; p=0.014) and lower maternal 25-OHD level (OR,1.16; 95 % 0,1.034-1.292; p=0.011).
Conclusions. Maternal/child VitD deficiency is associated with ID/IDA in children aged 6 months-5 years.
Key Words: Child; Iron deficiency; Vitamin D.
INTRODUCTION
Iron deficiency (ID) is the most common nutritional deficiency and health problem all over the world.1 It is a well-known problem, especially in children and women. The prevalence of anemia worldwide is 47.4 % in preschool children, 41.8 % in pregnant women and 30.2 % in nonpregnant women. In Turkey, ID and iron deficiency anemia (IDA) was estimated to be between 21 % and 35 % in different studies of children.2 According to the World Health Organization (WHO), if anemia prevalence in a country reaches approximately 40 %, it is a significant community health problem. Untreated IDA leads to growth retardation, mental and cognitive impairments, predisposition to infections, and increase in childhood mortality.
Vitamin D (VitD) is an essential nutrient that plays an important role in calcium homeostasis and bone health. 1,25(OH)2D is an important mediator of active calcium absorption from the intestine, and deficiency of VitD is known to cause rickets in growing children and osteomalacia in older adolescents and adults. Vitamin D is also important factor for the development of brain cells and axonal growth. Children are growing organisms, so its deficiency may cause certain disorders in children. Children with attention deficit/hyperactivity disorder (ADHD) were found to have lower level of VitD than normals.3 Also iron deficiency is associated with ADHD.4 Populations at increased risk include exclusively breastfed infants, particularly if the mother has been VitD deficient during pregnancy, dark-skinned children, those living at higher latitudes, and those with limited sun exposure for a multitude of reasons.
In chronic kidney disease, serum concentrations of the prohormone 25-hydroxy vitamin D (25-OHD) level is correlated inversely with the prevalence of anemia5 and erythropoiesis stimulating agent resistance6 and directly with blood hemoglobin levels.6 Bacchetta et al.,7 found that VitD is a potent regulator of hepsidin-ferroportin axis in humans. The binding of 1,25-dihydroxyvitamin D (calcitriol) to the VitD receptor decreased mRNA levels of hepcidin in vitro.7 In vitro studies of bone marrow red cells have demonstrated that calcitriol increases erythropoietin receptor expression and, synergistically with erythropoietin stimulates proliferation.8 The aim of this study was to investigate whether there was an association between maternal/child 25-OHD levels and ID in children aged 6 months to 5 years.
POPULATION AND METHODS
Between September 2014 and January 2016 children aged 6 months-5 years admitted to the outpatient clinic of Cumhuriyet University Faculty of Medicine Hospital Department of Pediatrics were added to the study. The exclusion criteria were birth history of prematurity (<37 weeks), presence of chronic illnesses, malnutrition, obesity, thalassemia trait, hemolytic anemia, macrositic anemia, acute infections or refusal of parental consent. The study protocol was approved by the Local Ethics Committee. Informed consent was obtained from the parents or legal guardian of the children. After a full physical examination, total blood count, serum iron level, total iron binding capacity (TIBC), serum ferritin level, erythrocyte sedimentation rate, 25-OHD, serum calcium, serum phosphorus and alkaline phosphatase levels were examined in the children and their mothers. All the measurements were made from venous blood specimens collected from the patients and mothers. The blood samples were taken in a fasting condition on the morning of admission. Empty tubes with gel were used for routine biochemistry analyses, and a tube containing K2EDTA was used for the complete blood count (all tubes from Becton Dickinson, Oxon, UK). Serum specimens were obtained after centrifugation of the blood samples. Routine biochemistry analyses and complete blood count tests were measured immediately. Serum iron and TIBC levels were determined in serum samples spectrophotometrically using an AU5800 auto analyzer (Beckman Coulter, USA). The leukocyte (WBC), platelet (Plt), hemoglobin (Hb), hematocrit (Htc), mean corpuscular volume (MCV), and red cell distribution width (RDW) were determined in whole blood samples using a hematology auto analyzer (Mindray BC 6800, China). 25-OHD levels were examined with the chemiluminescence immunoassay method (Beckman UniCel DxI 600, USA).
The children were classified as ID/IDA (Group 1) or normal (Group 2). Iron deficiency was defined as Hb level normal but ferritin level <12 ng/dl and transferrin saturation (serum iron/TIBC) <16 %.9 If the Hb level was <11 g/dl and MCV was <70 flt+age, together with ID, the patient was accepted as IDA.1,9 Vitamin D sufficiency was defined as 25-OHD level between 20 and 100 ng/mL (50-250 nmol/L). Vitamin D insufficiency was defined as 25-OHD level between <20 ng/ml and >12 ng/ml (30-50 nmol/L) and VitD deficiency was defined as 25-OHD level <12 ng/ml (<30 nmol/L).10 Maternal ID was defined as ferritin level<15 ng/ml and Hb level <12 g/dl.11 Erythrocyte sedimentation rate was considered high if it was >20 mm/h, and in this situation, the patient was not accepted in the study population.
In Turkey, iron supplementation is given as a preparation containing iron III hydroxide polymaltose in drop form, which has 2.5 mg elementary iron in one drop. This preparation is given by Family Health Centers to all infants aged 4-12 months. For full term infants, 1mg/kg elemental iron is given. In addition, VitD supplementation [400 international units (IU), one droplet of VitD preparation containing about 150 IU VitD] is given to all infants by Family Health Centers from birth to 12 months of age. For the purpose of this study, information was obtained from the mother through a questionnaire asking maternal age, duration of iron supplementation of the mother during pregnancy, the number of children the mother has previously given birth to, sibling ranking of the child, exclusively breastfeeding time, starting month of weaning period, starting month of iron supplementation of infant, duration (months) of iron supplementation of infant, number of drops of iron preparation given to infant, duration (months) of VitD supplementation during infancy and number of drops of VitD given.
All statistical analyses were performed using SPSS 15.0 software (SPSS, Chicago, IL, USA). The Student's t-test was used for normally distributed continuous variables. Categorical data were analyzed using the Chi square test and the Fisher exact test. The Pearson correlation coefficient was used to determine the relationship between Hb, ferritin levels and 25-OHD levels. To investigate independent risk factors for low Hb and ferritin levels in the children, multivariable logistic regression analysis was applied. The necessary sample size was calculated to be at least 50 individuals in each group, with 90 % power and 0.05 type I error, anticipation of a deviation of ± 20 % in the control group (R 3.0.1. open source program) depending on the literature data.12 A value of p< 0.05 was considered to be statistically significant.
RESULTS
A totally 2900 children were admitted to outpatient clinic from September 2014 to January 2016. Among them 2250 children had infection, 180 had malnutrition, 33 had chronic diseases, 45 had prematurity, 150 children were referred to other hospitals, 75 had other causes of anemia (vitamin B12 deficiency, thalassemia trait, hemolytic anemias, etc.). Parents of 50 children refused the involvement in the study. A total of 117 children were included in the study, comprising 67 in the ID/IDA group (Group 1) and 50 in the normal group (Group 2). There was no difference between Group 1 and 2 in respect of age, gender, birth week, weight and height (Table 1). The mothers in Group 1 were younger than Group 2 (Table 1, p=0.04) and they had more children than Group 2 (Table 1, p=0.001) with the child ranking higher among siblings in Group 1 than in Group 2 (2.24±1.03 vs. 1.72±0.88; p=0.006). The mothers in Group 2 were taken longer iron supplementation during pregnancy (Table 1, p=0.03). The mean duration of exclusively breastfeeding was longer in Group 1 than in Group 2 (Table 1; p=0.001). The children in Group 2 had longer iron supplementation and more number of drops given during infancy (Table 1, p=<0.001). The mean duration of VitD supplementation during infancy was longer in Group 2 than in Group 1(Table 1, p=0.003).
Table 1. The demographic characteristics of children in Group 1 (ID/IDA) and Group 2 (normal)

The comparisons of the hematological, and biochemical values of the children and mothers in the two groups are summarized in Table 2. 25-OH vitamin D levels were lower in Group 1 than in Group 2 (21.9±12 vs. 33.3±22, p=<0.001). The mothers of Group 1 had lower levels of Hb, Htc, MCV, ferritin and 25-OHD levels than the mothers of Group 2 (Table 2). Vitamin D insufficiency was determined in 33/67 (49.3 %) children and in 63/67 (94.0 %) mothers in Group 1 and in 10/50 (20.0 %) children and 32/50 (64.0 %) mothers in Group 2 (children: p=0.001, mothers: p=<0.001) (Table 3). There was a positive correlation between Hb levels of children and mean duration of regular iron supplementation during infancy, the number of drops of iron preparation given, child 25-OHD, mother 25-OHD, mother Hb and ferritin level (Table 4). There was a negative correlation between the Hb levels of children and number of children of the mother, the sibling ranking of the child, exclusively breastfeeding time, infant age at starting weaning (Table 4).
Table 2. The Laboratory Test Results of Group 1 (ID/IDA) and Group 2 (Normal)
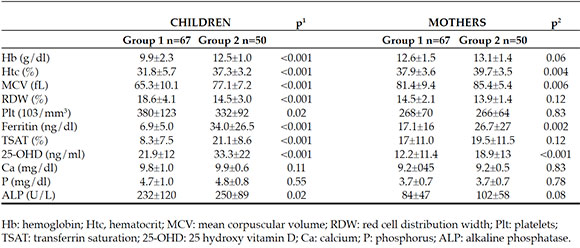
Table 3. Vitamin D deficiency distributions of mothers and children according to groups

Table 4. Correlations between some parameters and ferritin and hemoglobin levels of children

Multivariable logistic regression analysis determined the independent risk factors for anemia in children to be the longer duration of exclusively breastfeeding (p=0.11), shorter duration of regular iron supplementation during infancy (p=0.008), lower levels of maternal 25-OHD (p=0.011), shorter duration of maternal iron supplementation during pregnancy (p=0.014) and later starting weaning period (p=0.006) (Table 5).
Table 5. Determinants of low hemoglobin and low ferritin levels in children
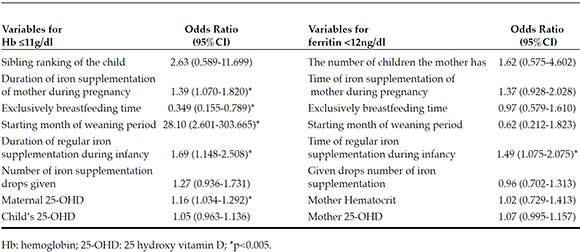
The only independent risk factor for low ferritin levels in children was determined to be the shorter duration of regular iron supplementation during infancy (p=0.017) (Table 5).
DISCUSSION
All exclusively breastfed infants should receive 400 IU per day of VitD supplements, beginning a few days after birth according to the Pediatric Endocrinology Society.10,13 In Turkey VitD deficiency as rickets is not frequent, the prevalence is 0.1 %14 although VitD deficiency is seen in 40 % of children.15 In a study from Turkey, maternal VitD deficiency was reported to be approximately 80 %.16 Under a Ministry of Health program, an iron preparation containing elementary iron 40-60 mg/day is given to pregnant women in Turkey for 6 months from the beginning of the 4th month (second trimester) to the 9th month of pregnancy and for 3 months after the birth for a total of 9 months but VitD supplementation is not routinely given to pregnant women. In the current study, a positive correlation between Hb/ferritin levels of children and children/maternal 25-OHD levels and also maternal Hb/ferritin levels were determined. In IDA/ID group (Groupl) there were more VitD insufficient+deficient mothers and children than in the normal group. This shows that children who have mothers with VitD deficiency are more likely to have ID and children with VitD deficiency are more likely to have ID. A total of 81 % of all the mothers had VitD deficiency, which was consistent with previous reports in literature.16 It is also known that maternal ID during pregnancy increases the risk of ID in the infant.17 In the current study consistent with findings in literature, low levels of maternal Hb and ferritin were observed more frequently in Group 1 than in Group 2 and iron supplementation during pregnancy was determined to be an independent risk factor for anemia in children.
In a study conducted on 10 410 children and adolescents in United States, low 25-OHD levels were associated with an increased risk of anemia.18 In Korea, Lee et al.,12 found that VitD deficiency is associated with increased risk of anemia especially IDA, in healthy female, children and adolescents. In a study by Jin et al.,19 under the age of 24 months VitD deficiency was reported to be more frequent in ID patients and there was a significant association between Hb and 25-OHD levels in infants. In the current study, a positive correlation was also found between Hb and 25-OHD levels of children under the age 5 years. Kang et al.,20 found that infants with anemia and their mothers had lower levels of VitD than those without anemia. However, in that study, 25-OHD levels were not associated as independent risk factors for iron deficiency in infants.20 In our study, there was a positive correlation between, child/mother 25-OHD and the hemoglobin/ferritin levels of children also the duration of VitD supplementation was longer in the Group 2. Usage of iron and VitD supplementations during infancy were the predictors of the ID/IDA of children also. The maternal 25-OHD level was an independent risk factor for anemia in children. Vitamin D deficiency in children and their mothers may have been an etiological factor for iron deficiency in children; it may have decreased iron absorption by increasing the hepcidin levels in these individuals7 or decreased erythropoietin receptor expression on stem cells.8 Also iron is the essential element for the functioning of cytochromes P450. Some of these cytochromes (CYP27A1, CYP24A1) play important role at the hydroxylation of VitD. Iron deficiency may affect the function of these enzymes and may cause VitD deficiency.21 The findings of this study explain that VitD deficiency of mothers and children affects the iron deficiency in children. However, further studies are needed to determine whether VitD deficiency is the cause of ID/IDA or iron deficiency is the cause of VitD deficiency or they are concomitant nutritional deficiencies. However according to our findings, we can suggest mothers should take iron and VitD supplementation during pregnancy; iron and VitD supplementation in infancy should be regular and under the regular control of health professionals.
In our study, the duration of exclusively breastfeeding was longer in the ID/IDA group. Some mothers fed their infant only with breastfeeding for 12 months. There was a negative correlation between this time and Hb/ferritin level in children. There was also a negative correlation between the ages of starting the weaning period. The time of starting the weaning period was seen to be later in Group 1. Thus, when the duration of exclusively breastfeeding is prolonged, the children are more likely to have ID. This finding is consistent with literature.22 In Turkey; the Ministry of Health recommends exclusively breastfeeding for 6 months, in accordance with WHO guidelines, and then starting iron rich complementary foods in addition to breastfeeding.
In our study, another interesting finding is that the maternal age was younger in Group 1 than in Group 2 and the mothers of Group 1 had more children than the mothers of Group 2. Young and inexperienced mothers with a large number of children increased the risk of ID in children. In the developing countries like Turkey the maternal age and education are important issues for the child and infant health. Our findings also support this. In the light of these results, it can be concluded that it is necessary to educate mothers especially young and inexperienced mothers about the importance of iron rich complementary foods should be started at the right time to prevent ID.
There are some limitations of our study. We obtained the data from the mothers when their children were 2, 3 or 4 years old and it might cause a memory bias.
CONCLUSION
Maternal/child VitD deficiency is associated with ID/IDA in children aged 6 months-5 years. However, further studies are needed to determine whether VitD deficiency is the cause of ID/IDA or a concomitant nutritional deficiency.
2. Aydin A, Gur E, Erener-Ercan T, Can G, et al. Comparison of different iron preparations in the prophylaxis of iron-deficiency anemia. J Ped Hematol Oncol. 2017; 39(7):495-9. [ Links ]
3. Villagomez A, Ramtekkar U. Iron, Magnesium, Vitamin D, and Zinc Deficiencies in Children Presenting with Symptoms of Attention-Deficit/Hyperactivity Disorder. Children (Basel). 2014; 1(3):261-79. [ Links ]
4. Wang Y, Huang L, Zhang L, Qu Y, et al. Iron Status in Attention-Deficit/Hyperactivity Disorder: A Systematic Review and Meta-Analysis. PLoS One. 2017; 12(1):e0169145. [ Links ]
5. Lac PT, Choi K, Liu IA, Meguerditchian S, et al. The effects of changing vitamin D levels on anemia in chronic kidney disease patients: A retrospective cohort review. Clin Nephrol. 2010; 74(1):25-32. [ Links ]
6. Kiss Z, Ambrus C, Almasi C, Berta K, et al. Serum 25(OH)-cholecalciferol concentration is associated with hemoglobin level and erythropoietin resistance in patients on maintenance hemodialysis. Nephron Clin Pract. 2011; 117(4):c373-8. [ Links ]
7. Bacchetta J, Zaritsky JJ, Sea JL, Chun RF, et al. Suppression of iron-regulatory hepcidin by vitamin D. J Am SocNephrol. 2014; 25(3):564-72. [ Links ]
8. Alon DB, Chaimovitz C, Dvilansky A, Lugassy G, et al. Novel role of 1,25(OH)2D3 in induction of erythroid progenitor cell proliferation. Exper Hematol. 2002; 30(5):403-9. [ Links ]
9. Lanzkowsky P. Iron deficiency anemia. In: Lanzkowsky P, Lipton J, Fish J (eds). Lanzkowsky's Manual of Pediatric Hematology and Oncology. 6th ed. San Diego, CA: Elsevier; 2016.Págs.69-83.
10. Misra M, Pacaud D, Petryk A, Collett-Solberg PF, et al. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008; 122(2):398-417. [ Links ]
11. Guyatt GH, Oxman AD, Ali M, Willian A, et al. Laboratory diagnosis of iron deficiency anemia: an overview. J Gen Intern Med. 1992; 7(2):145-53. [ Links ]
12. Lee JA, Hwang JS, Hwang IT, Kim DH, et al. Low vitamin D levels are associated with both iron deficiency and anemia in children and adolescents. Pediatr Hematol Oncol. 2015; 32(2):99-108. [ Links ]
13. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011; 96(7):1911-30. [ Links ]
14. Ozkan B, Doneray H, Karacan M, Vançelik S, et al. Prevalence of vitamin D deficiency rickets in the eastern part of Turkey. Eur J Pediatr. 2009; 168(1):95-100. [ Links ]
15. Andiran N, Çelik N, Akça H, Dogan G. Vitamin D deficiency in children and adolescents. J Clin Res Pediatr Endocrinol. 2012; 4(1):25-9. [ Links ]
16. Erol M, İşman FK, Kucur M, Hacibekiroglu M. Annede D vitamin eksikliginin degerlendirilmesi. Türk Pediatri Ars¸. 2007; 42(1):29-32. [ Links ]
17. Kumar A, Rai AK, Basu S, Dash D, et al. Cord blood and breast milk iron status in maternal anemia. Pediatrics. 2008; 121(3):e673-7. [ Links ]
18. Atkinson MA, Melamed ML, Kumar J, Roy C, et al. Vitamin D, race, and risk for anemia in children. J Pediatr. 2014; 164(1):153-8.e1. [ Links ]
19. Jin HJ, Lee JH, Kim MK. The prevalence of vitamin D deficiency in iron-deficient and normal children under the age of 24 months. Blood Res. 2013; 48(1):40-5. [ Links ]
20. Kang YS, Kim JH, Ahn EH, Yoo EG, et al. Iron and vitamin D status in breastfed infants and their mothers. Korean J Pediatr. 2015; 58(8):283-7. [ Links ]
21. Nebert DW, Wikvall K, Miller WL. Human cytochromes P450 in health and disease. Philos Trans R Soc Lond B Biol Sci. 2013; 368(1612):20120431. [ Links ]
22. Hong J, Chang JY, Shin S, Oh S. Breastfeeding and red meat intake are associated with iron status in healthy Korean weaning-age infants. J Korean Med Sci. 2017; 32(6):974-84. [ Links ]











 texto en
texto en 


