Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos argentinos de pediatría
versión impresa ISSN 0325-0075versión On-line ISSN 1668-3501
Arch. argent. pediatr. vol.117 no.5 Buenos Aires oct. 2019
http://dx.doi.org/10.5546/aap.2019.306
ORIGINAL ARTICLE
http://dx.doi.org/10.5546/aap.2019.eng.306
Is there an association between vitamin D levels and cow's milk protein allergy at infancy?
Nazli Ercan, M.D.a, Prof. ilknur B. Bostanci', Prof. Serap Ozmen, M.D.b and Mustafa A. Tekindal, ph.D.c
a. Health Sciences University, Gulhane Training and Research Hospital, Department of Pediatric Immunology and Allergy, Ankara, Turkey.
b. Dr. Sami Ulus Obstetrics, Children's Health and Diseases Training and Research Hospital, Department of Pediatric Immunology and Allergy, Ankara, Turkey.
c. Başkent University, School of Medicine, Department of Biostatistics, Ankara, Turkey.
E-mail address: Nazli Ercan, M.D.: drnazliercan@gmail.com
Funding: None.
Conflict of interest: None.
Received: 7-29-2018
Accepted: 3-11-2019
ABSTRACT
Aim. Food allergy is an important public health concern with an increasing prevalence. The objectives were to determine the possible association between cow's milk protein allergy (CMPA) and 25(OH)D (vitamin-D) levels of infants with an initial diagnosis of CMPA and the association between 25(OH)D levels and (a) SPT (skin prick test) induration size, (b) specific IgE to milk, (c) specific IgE to casein.
Methods. Prospective, observational, case control study; the study group was composed of children < 2 years of age with a diagnosis of CMPA confirmed by an oral food challenge test. Healthy infants were enrolled as controls. Serum 25(OH) D levels were obtained at the initial workup in both groups. The correlation of vitamin-D levels was investigated in the development of CMPA.
Results. Among the study group of patients (n:56) 41,1% (n:23) had IgE-mediated and 58,9% (n:33) had non-IgE-mediated allergies There were no statistically significant differences between the CMPA and control groups (n: 55), in terms of serum 25(OH)D levels (33.85 ± 16.18ng/ml, 30.70 ± 14.90ng/ml; respectively, p:0.289). No statistically significant difference was found between study and controls according to 25(OH) D levels (adequate, insufficiency, deficiency; p=0.099). In the SPT of the CMPA group, a negative weak correlation without statistical significance was determined between the induration diameter of milk antigen and serum levels of 25(OH)D (p:0,794; r= -0,037).
Conclusions. No significant difference was found in serum 25 (OH)D levels between infants with CMPA and healthy controls. Our results do not support the routine request for 25(OH)D levels in pediatric age CMPA patients at their initial workup.
Key words: Milk hypersentivity; Food hypersentivity; Vitamin D.
INTRODUCTION
Food allergies constitute the majority of allergic reactions in early childhood. Its prevalence in the pediatric age group (especially in patients under the age of 3 years) is 6 %, in comparison to 3.5-4 % in the general population.1 The most frequent causes of food allergies vary between geographic regions, population genetics, food cultures in particular methods of cooking and the introduction of new foods in infants.2 Cow's milk protein (CMP), egg, soy, peanut, and wheat flour are the major causes (80 %) of food allergies. Cow's milk protein allergy (CMPA) is an important health concern particularly in the first two years of life and is one of the most commonly encountered food allergies.3 A possible explanation for food-related reactions may be the rise in vitamin-D -25(OH)D-deficiency at childhood, which is suggested to be synchronous with food allergy.4
The first etiological clues supporting the association between food allergies and vitamin-D deficiency arose from the reported differences in EpiPen (epinephrine injection, USP) prescriptions between the northern and southern regions of United States, suggested to be due to differences in ultraviolet B exposure.5 The same findings have been replicated and extended to anaphylaxis admissions to emergency departments in different latitudes of Australia.6 Osborne NJ et al. reported that the prevalence of both food allergy and eczema, but not asthma, is strongly related to the latitude of residence. Their study supported the hypothesis that solar irradiance and its associated effects
on vitamin-D status might play a role in the increasing prevalence of food allergy and eczema. Latitude differences were also shown in food allergy prevalence studies.7 Furthermore, the seasonal differences in exposure to UV rays were suggested to contribute to the development of food allergies in early childhood. Recent studies have shown that patients with food allergy often have low levels of ambient UV and consequently have lower sources of vitamin-D who especially those born in the autumn/winter period.8,9
Vitamin-D deficiency may result in the breakdown of such defenses as cathelicidins or mucosal barrier function resulting in abnormal microbial flora. This could lead to the development of gastrointestinal tract infections, as a result of which the immune system could easily be exposed to food allergens. Therefore with the breakdown of immune tolerance and other possible accompanying effects of vitamin-D deficiency on immune response, it could further provoke food allergies.10
In this study, we hypothesized that vitamin-D may have a role in the pathogenesis of CMPA. Our main objective was to find out the possible association between 25(OH)D levels and infants who suffer from CMPA at their initial diagnosis. The secondary objective was to determine the association between 25(OH)D levels and (a) SPT (Skin Prick Test) induration size, (b) specific IgE to milk, (c) specific IgE to casein.
POPULATION AND METHODS
This case-control study was carried out at Pediatric Immunology and Allergy Department of Dr. Sami Ulus Obstetrics, Children's Health and Diseases Training and Research Hospital, Ankara, Turkey over a one-year period. The trial was authorized (Approval Number: 223-2013, http://akeah.gov.tr) by the ethical committee of clinical research centers' of the Turkish Ministry of Health. Written informed consent was obtained from families of all infants before enrollment to the study.
The inclusion criteria of the study group were; infants less than 2 years of age, at their first admission to the Pediatric Immunology and Allergy department with suspected CMPA. Among those 92 infants, 56 were enrolled in the study group who received the definitive CMPA diagnosis with the oral food challenge. The control group (n= 55) were enrolled from our healthy-child-outpatient unit, from infants who were admitted for a routine check-up without an allergic disease history. To ensure the homogeneity of the groups the seasonal birth times of the controls were matched to the study patients. No patient required hospitalization in the study.
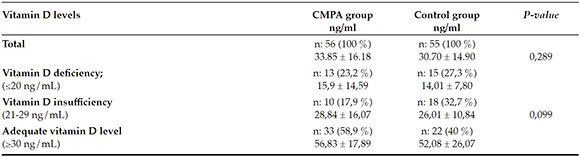
All infants who were known to suffer from chronic diseases (i.e.; chronic lung disease, congenital heart disease, chronic kidney disease, immune deficiency and malnutrition, etc.) and children who are receiving elimination diet with suspected CMPA (due to the possibility of existing vitamin-D deficiency) or who had already been diagnosed with CMPA were not enrolled to the study. The study and control groups' initial data were collected mainly focusing on these items: (1) demographic data; (2) serum 25(oh)d, calcium, phosphorus, alkaline phosphatase, serum total ige levels, and (3) spt (skin prick test) results. At the time of the diagnosis all serum samples were studied with chemiluminescence immunoassay method for 25(OH)D levels with the analyzer; SHIMADZU-API LC-MS-API 32000. As described by Holick et al.,11 vitamin-D levels were considered deficient when 25(OH)D levels were ≤20 ng/mL; insufficient 21 to 29 ng/mL; and sufficient ≥30 ng/mL.
The SPT were applied directly into the skin of the back or forearm at regular two centimeter intervals by stallerpoint (Stallergenes®) with the antigens of milk, egg yolk, egg white, wheat flour, cat, potato, negative control and histamine (ALK, Abello, Copenhagen, Denmark), and prick to prick fresh milk. As an aeroallergen, only cat epithelial antigen was used with its high allergenic activity.12 The test was evaluated 20 minutes later and an induration size of >3 mm was accepted as positive.
The definitive diagnosis of CMPA was performed in steps of clinical history, physical examination and finally oral food challenge test (OFC) as described previously.13,14 Thus in turn to the whole study group; (1) SPT, (2) Cow's milk-specific IgE and casein-specific IgE (for both parameters a value of > 0,35 kU/L was accepted as positive; Dr Fooke-Achterrath Laboratorien GmbH HAbicktweg 16, D41468 Neuss, Germany), (3) diagnostic elimination diet and finally (4) OFC was performed. The OFC was contraindicated in the case of a history of anaphylaxis within one year which was confirmed by a positive SPT and/or positive specific IgE result, and also in infants with malnutrition as a late manifestation of CMPA. The diagnosis of multiple food allergy was confirmed by the OFC test to all other suspicious foods, in patients with cow's milk allergy. Patients who were diagnosed with CMPA were divided into two subgroups as already described previously by Allen KJ et al.;15 Ig E mediated (n= 23; 41,1 %) (includes: urticaria, angioedema, anaphylaxis, wheezing and conjunctivitis) or non-Ig E mediated (n= 33; 58,9 %) (includes: atopic dermatitis, food protein-induced enteropathy, enterocolitis and proctocolitis).
Firstly, study and control groups' 25(OH)D levels at the time of diagnosis were compared and its relationship with CMPA development was statistically evaluated. Secondly, the subgroup analysis was performed (IgE and non-IgE-mediated CMPA subgroups). In this time-limited study since the seasonal differences might lead to a bias, the number of children was equally distributed to the study and control groups according to the seasons. We initially calculated the least sample size of individuals as 92 for the study with 80 % power and at the p=.05 significance level. SPSS for Windows, version 15.0 (SPSS, Inc., Chicago, IL) was used for statistical analysis. The one-sample Kolmogorov-Smirnov test was used to analyze the normality for continuous variables. The Mann-Whitney U test was used for comparison of the continuous variables. Data were described as median (min-max) and mean ± standard deviation, number, and percentage according to the type of the variable. For correlation analyses, Spearman's correlation analysis test was used.
RESULTS
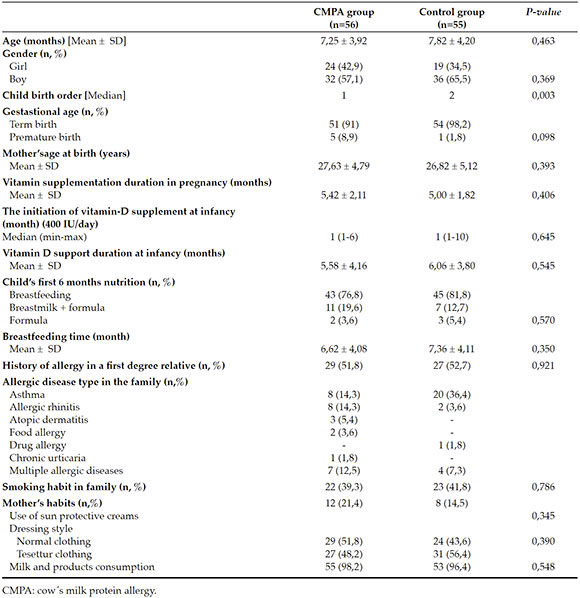
The flow chart of the study is given in Figure 1 and the demographic and clinical parameters of the study and control groups are given at Table 1. There were no significant differences between the study and control groups due to the independent risk factors that could possibly have an effect on vitamin-D levels of infants. Some of those are; mothers' clothing style (p: 0,390), the use of sun protective creams (p: 0,345), duration of vitamin D supplementation during pregnancy (p: 0,406) or at infancy (p: 0,545), breastfeeding time (p: 0,350). As it was expected, CMPA was more frequently observed in the first child of the families of the study group (p: 0.003) (Table 1).
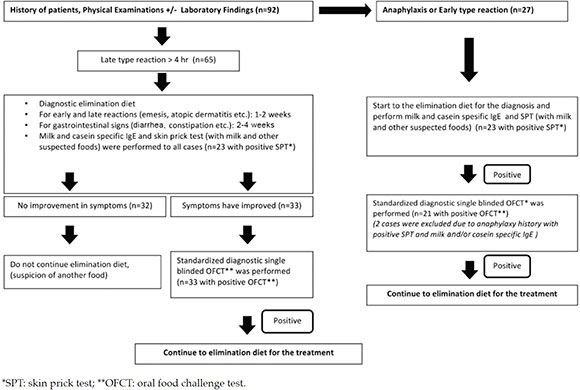
Figure 1. Flowchart of the study
Table 1. The demographic characteristics and clinical parameters of the study
There were no significant statistical differences between the CMPA and control groups, in terms of initial diagnostic serum 25(OH)D levels (33.85 ± 16.18 ng/ml, 30.70 ± 14.90 ng/ml respectively; p= 0.289). In addition, no statistically significant difference was found among the groups in regard to serum calcium, phosphorus, and alkaline phosphatase levels (p= 0.403, p= 0.300, and p= 0.369; respectively) (Table 2). Both groups' 25(OH)D vitamin level classifications are given at Table 3. No statistically significant difference was found between the CMPA and control groups in terms of the vitamin-D classification system (p= 0.099) (Table 3).
Table 2. The laboratory results of CMPA and control groups
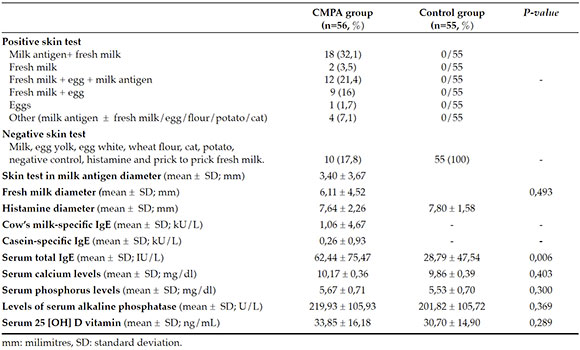
Table 3. Vitamin D level classifications of CMPA and control groups
The diagnosis of patients, of the CMPA group, was mostly constituted of infants with atopic dermatitis (n: 27, 48 %). This was followed by 23 patients (41,1 %) who had IgE mediated diseases (urticaria, angioedema, anaphylaxis, wheezing and conjunctivitis). No statistically significant differences were found between the diagnostic subgroups of CMPA (Ig E mediated and non-Ig E mediated) in terms of 25(OH) levels (p= 0.587). The infants who were allergic to CMP (n: 20), infants presented with proctocolitis without SPT positivity (n: 10) and infants who were both allergic to CMP and had multiple food allergy (n: 26) were compared according to their 25(OH)D levels. No statistically significant difference was found between groups (p= 0.766).
Other objectives of the study were to determine the association between serum 25(OH)D levels and (a) SPT (skin prick test) induration size, (b) specific IgE to milk, (c) specific IgE to casein in infants who were allergic to CMP. In the study group, a negative weak correlation without statistical significance was found between vitamin-D levels and the induration diameter of SPT with milk antigens (p= 0.794) (r= -0,037). Furthermore, no significant correlation was found between serum 25(OH)D levels and the induration diameter of fresh cow's milk (p= 0.902; r= -0,017). No significant correlation was found between milk-specific IgE and 25(OH)D levels (p= 0.47; r= +0,1). A-negative weak correlation was detected between casein-specific IgE and 25(OH)D levels (p: 0,706; r= -0,052).
DISCUSSION
There was no statistically significant difference in terms of vitamin-D levels between the study and control groups. When serum 25(OH)D levels were classified into vitamin-D insufficiency, deficiency or adequate, no significant differences were found between CMPA and control groups. Both our study and control groups were investigated according to possible independent risk factors that may have roles in the development of CMPA. Except for the birth order of infants, all the variables were similar between the study and control groups.
There are few published studies in the literature, investigating the relationship between vitamin-D levels and food sensitization or food allergy.16-24 According to National Health and Nutrition Examination Survey (NHANES) results, after multivariate adjustment, peanut sensitization defined by specific IgE titration were twice more likely in children/adolescents whose 25(OH)D levels were <15 ng/mL in comparison to 25(OH)D levels of ≥30 ng/mL.20 However, in the same study, no significant association was reported between sensitization to cow's milk and vitamin-D levels. Moreover, no significant correlation was reported between levels of vitamin-D and allergic symptoms like eczema. The investigators concluded that vitamin-D deficiency is associated with higher levels of IgE sensitization in children and adolescents, and pointed out further trials on the same topic.20
Another evidence of a connection between vitamin-D deficiency and food allergy was recently published from a Melbourne/Australian, population-based cohort study with the enrollment of babies under the age of 1.22 Infants of Australian-born parents, but not of parents born overseas, with vitamin-D insufficiency (≤50 nmol/L) were more likely to be peanut (P=.006) and/or egg (P =.025) allergic than were those with adequate vitamin-D levels, independent of their eczema status. Among those with Australian-born parents, infants with vitamin-D insufficiency were twice more likely to have multiple food allergies rather than a single food allergy. Another interesting outcome was of the infants who were food sensitized. Vitamin-D insufficient infants were six times more likely to be food allergic than tolerant. The authors concluded that these results could provide evidence of the sufficiency of vitamin-D may be an important protective factor for food allergy at the infancy period.22
A recently published study about atopic dermatitis or suspected food allergy in infants had shown that low vitamin-D levels are associated with enhanced food sensitization. In particular, it was demonstrated that low vitamin-D levels can lead to a 10-fold increase in sensitization to CMP.23 However, in this study food allergy group was defined as suspicious and the diagnosis didn't proved by oral food challenge test. A-strength of our study was the gold standard diagnostic technique; OFC performed to all study group of infants at the accurate diagnosis of CMPA.14
In the literature, studies investigating the relationship between food allergy and vitamin-D levels mainly focused on atopic dermatitis.1619,20,23-25 Our study differs in a way that, CMPA group included not only atopic dermatitis but also other non-IgE-mediated clinical presentations of CMPA such as food protein-induced enteropathy, enterocolitis and proctocolitis. However, in line with published data, our CMPA group was mostly constituted of atopic dermatitis and almost half of them had adequate serum vitamin D levels.
In a recently published meta-analysis no significant association between the status of vitamin-D and IgE mediated food allergy was reported.26 In furtherance, we identified no statistically significant correlation between vitamin-D levels and both CMPA diagnostic groups (IgE and non-IgE-related). The interpretation of two case-control, two crosssectional, and one prospective cohort study at the meta-analysis was limited by a lack of a standard definition for the vitamin-D deficiency. The authors also pointed out the necessity of finding the optimal threshold of vitamin-D levels to maintain the immune function in preventing the body from atopic diseases.26
In the literature infants who have multiple allergen sensitizations in SPT with atopic eczema or food allergy, 25(OH)D levels were found to be significantly lower than those of patients who had monosensitization in SPT.24 In our study, 20 of CMPA patients had sensitization to only cow's milk in SPT, whereas 26 patients had also sensitization to other allergens (egg, wheat flour, potato and cat antigens). Conversely, when our CMPA group's serum 25(OH)D levels were compared between only cow's milk or multiple allergen sensitizations (as determined by SPT), no significant correlation was found.
There is a known relation between CMPA symptom severity and SPT induration diameter and specific IgE levels.27 We found no statistically significant correlation between milk antigen induration diameter and vitamin-D levels of CMPA infants. Likewise, no significant correlation was recorded between 25(OH)D levels and induration diameter of fresh milk, cow's milk and casein-specific IgE. This could be due to our relatively low number of patients enrolled and was assumed to be a limitation of the trial.
A-strengthening point was the birth seasons of our patients which were evenly distributed among four seasons, the patients' characteristics including all of the clinical forms of CMPA, and the accurate patient diagnosis with oral food challenge test in all participants. In considering a study investigating the association with food allergy and serum vitamin-D levels, supplementation of mothers and infants or enrichment of nutrition with vitamin-D could make the research more complicated.28 Likewise, maternal vitamin-D intake could also have a protective, adjuvant effect or vice versa on food allergy.20,29-31 In Turkey as a preventive health care policy and recommended by American and European Academy of Pediatric guidelines,32,33 the estimated daily requirement of vitamin-D supplementation is routinely initiated at the second trimester of all pregnancies (500 IU/day) and to all newborns (400 IU/day) at their infancy. Therefore in our study, all mothers and infants had received vitamin-D supplementation during their pregnancy and infancy periods. We strictly documented the duration and the dosage of vitamin-D used during pregnancy and at infancy, to standardize patients and avoidance of bias.
In conclusion, no statistically significant relationship was found between the CMPA group and healthy controls in terms of 25 (OH) D levels. There is still a need for further prospective, multicenter trials in investigating the optimal status of vitamin-D in CMPA. However, we may suggest that at the initial diagnosis of infants with CMPA, routine work-up of vitamin-D levels have no benefit.
1. Nowak-Węgrzyn A, Wesley Burks A, Sampson HA. Reactions to Foods. In: Adkinson N Jr, Bochner B, Burks A, BuseW, et al (eds). Middleton's Allergy Principles and Practice. 8th ed. Philadelphia: Elsevier; 2013. p. 1310-35. [ Links ]
2. Shek LP, Lee BW. Food allergy in Asia. Curr Opin Allergy Clin Immunol. 2006; 6(3):197-201. [ Links ]
3. Sampson HA. Legumes, eggs, and milk. Allergy. 1998; 53(46 Suppl):38-43. [ Links ]
4. Jones AP, Tulic MK, Rueter K, Prescott SL. Vitamin D and allergic disease: Sunlight at the end of the tunnel? Nutrients.2012; 4(1):13-28. [ Links ]
5. Camargo CA Jr, Clark S, Kaplan MS, eLieberman P, Wood RA. Regional differences in EpiPen prescriptions in the United States: the potential role of vitamin D. J Allergy Clin Immunol. 2007; 120(1):131-6. [ Links ]
6. Mullins RJ, Clark S, Camargo CA Jr. Regional variation in epinephrine autoinjector prescriptions in Australia: more evidence for the vitamin D-anaphylaxis hypothesis. Ann Allergy Asthma Immunol. 2009; 103(6):488-95. [ Links ]
7. Osborne NJ, Ukoumunne OC, Wake M, Allen KJ. Prevalence of eczema and food allergy is associated with latitude in Australia. J Allergy Clin Immunol. 2012; 129(3):865-7. [ Links ]
8. Mullins RJ, Clark S, Katelaris C, Smith V, et al. Season of birth and childhood food allergy in Australia. Pediatr Allergy Immunol. 2011; 22(6):583-9. [ Links ]
9. Keet CA, Matsui EC, Savage JH, Neuman-Sunshine DL, et al. Potential mechanisms for the association between fall birth and food allergy. Allergy. 2012; 67(6):775-82. [ Links ]
10. Roider E, Ruzicka T, Schauber J. Vitamin D, the cutaneous barrier, antimicrobial peptides and allergies: is there a link? Allergy Asthma Immunol Res. 2013; 5(3):119-28. [ Links ]
11. Holick MF. Vitamin D deficiency. N Engl J Med. 2007; 357(3):266-81. [ Links ]
12. Bonnet B, Messaoudi K, Jacomet F, Michaud E, et al. An update on molecular cat allergens: Fel d 1 and what else? Chapter 1: Fel d 1, the major cat allergen. Allergy Asthma Clin Immunol. 2018; 14:14. [ Links ]
13. Koletzko S, Niggemann B, Arato A, Dias JA, et al. Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI Committee Practical Guidelines. J Pediatr Gastroenterol Nutr. 2012; 55(2):221-9. [ Links ]
14. Fiocchi A, Brozek J, Schünemann H, Bahna SL, et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow's Milk Allergy (DRACMA) Guidelines. World Allergy Organ J. 2010; 3(4):57-161. [ Links ]
15. Allen KJ, Hill DJ, Heine RG. 4. Food allergy in childhood. Med J Aust. 2006; 185(7):394-400. [ Links ]
16. Oren E, Banerji A, Camargo CA Jr. Vitamin D and atopic disorders in an obese population screened for vitamin D deficiency. J Allergy Clin Immunol. 2008; 121(2):533-4. [ Links ]
17. Mullins RJ, Clark S, Wiley V, Eyles D, Camargo CA Jr. Neonatal vitamin D status and childhood peanut allergy: a pilot study. Ann Allergy Asthma Immunol. 2012; 109(5):324-8. [ Links ]
18. Rothers J, Wright AL, Stern DA, Halonen M, Camargo CA Jr. Cord blood 25-hydroxyvitamin D levels are associated with aeroallergen sensitization in children from Tuscon, Arizona. J Allergy Clin Immunol. 2011; 128(5):1093-9.e1-5. [ Links ]
19. Jones AP, Palmer D, Zhang G, Prescott SL. Cord blood 25-Hydroxyvitamin D3 and allergic disease during infancy. Pediatrics. 2012; 130(5):e1128-35. [ Links ]
20. Sharief S, Jariwala S, Kumar J, Muntner P, Melamed ML. Vitamin D levels and food and environmental allergies in the United States: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2011; 127(5):1195-202. [ Links ]
21. Weisse K, Winkler S, Hirche F, Herberth G, et al. Maternal and newborn vitamin D status and its impact on food allergy development in the German LINA cohort study. Allergy. 2013; 68(2):220-8. [ Links ]
22. Allen KJ, Koplin JJ, Ponsonby AL, Gurrin LC, et al. Vitamin D insufficiency is associated with challenge-proven food allergy in infants. J Allergy Clin Immunol. 2013; 131(4):1109-16. [ Links ]
23. Peroni DG, Piacentini GL, Cametti E, Chinellato I, Boner AL. Correlation between serum 25-hydroxyvitamin D levels and severity of atopic dermatitis in children. Br J Dermatol.2011; 164(5):1078-82. [ Links ]
24. Baek JH, Shin YH, Chung IH, Kim HJ, et al. The link between serum vitamin D level, sensitization to food allergens, and the severity of atopic dermatitis in infancy. J Pediatr. 2014; 165(4):849-54.e1. [ Links ]
25. Sidbury R, Sullivan AF, Thadhani RI, Camargo CA Jr. Randomized controlled trial of vitamin D supplementation for winter-related atopic dermatitis in Boston: a pilot study. Br J Dermatol. 2008; 159(1):245-7. [ Links ]
26. Willits EK, Wang Z, Jin J, Patel B, et al. Vitamin D and food allergies in children: A systematic review and metaanalysis. Allergy Asthma Proc. 2017; 38(3):21-8. [ Links ]
27. Luyt D, Ball H, Makwana N, Green MR, et al. BSACI guideline for the diagnosis and management of cow's milk allergy. Clin Exp Allergy. 2014; 44(5):642-72. [ Links ]
28. Rochat MK, Ege MJ, Plabst D, Steinle J, et al. Maternal vitamin D intake during pregnancy increases gene expression of ILT3 and ILT4 in cord blood. Clin Exp Allergy.2010; 40(5):786-94. [ Links ]
29. Nwaru BI, Ahonen S, Kaila M, Erkkola M, et al. Maternal diet during pregnancy and allergic sensitization in the offspring by 5 yrs of age: a prospective cohort study. Pediatr Allergy Immunol. 2010; 21(1 Pt 1):29-37. [ Links ]
30. Jones AP, Palmer D, Zhang G, Prescott SL. Cord blood 25-hydroxyvitamin D3 and allergic disease during infancy. Pediatrics. 2012; 130(5):e1128-35. [ Links ]
31. Weisse K, Winkler S, Hirche F, Herberth G, et al. Maternal and newborn vitamin D status and its impact on food allergy development in the German LINA cohort study. Allergy. 2013; 68(2):220-8. [ Links ]
32. Ahrens KA, Rossen LM, Simon AE. Adherence to Vitamin D Recommendations Among US Infants Aged 0 to 11 Months, NHANES, 2009 to 2012. Clin Pediatr (Phila). 2016; 55(6):555-6. [ Links ]
33. Grossman Z, Hadjipanayis A, Stiris T, Del Torso S, et al. Vitamin D in European children-statement from the European Academy of Paediatrics (EAP). Eur J Pediatr.2017; 176(6):829-31. [ Links ]











 texto en
texto en 


