Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Archivos argentinos de pediatría
versão impressa ISSN 0325-0075versão On-line ISSN 1668-3501
Arch. argent. pediatr. vol.117 no.6 Buenos Aires dez. 2019 Epub 01-Dez-2019
http://dx.doi.org/10.5546/aap.2019.e557
Original articles
Acute kidney injury in children after cardiac surgery: Risk factors and outcomes. A retrospective cohort study
aDepartment of Pediatrics, Pediatric Clinical Medicine. Hospital Británico de Buenos Aires, Autonomous City of Buenos Aires
bDepartment of Pediatrics, Pediatric Nephrology. Hospital Británico de Buenos Aires, Autonomous City of Buenos Aires
cDepartment of Pediatrics, Pediatric Cardiology. Hospital Británico de Buenos Aires, Autonomous City of Buenos Aires
dDepartment of Pediatrics, Pediatric Intensive Care Unit. Hospital Británico de Buenos Aires, Autonomous City of Buenos Aires
Introduction
Acute kidney injury (AKI) has been described as a common complication ofcardiac surgery in pediatric patients, whoseimpact on morbidity and mortality has beendocumented. Its incidence has been estimated tobe approximately 40 % in this patient group. Theobjective of this study was to estimate the incidenceof AKI in patients who underwent cardiovascularsurgery and to define associated risk factors andthe impact of AKI on the parameters of the postoperative course.
Population and methods
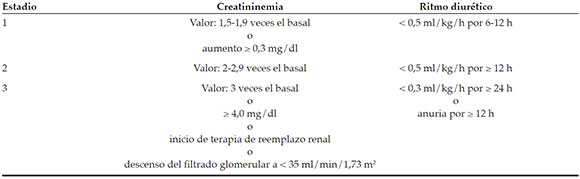
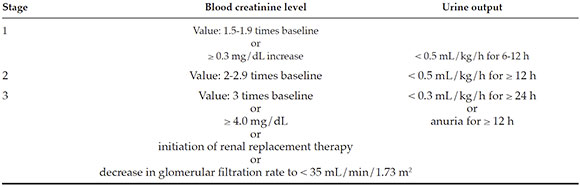
This was a retrospective, observational study of pediatricpatients who underwent cardiovascularsurgery between January 2015 and December2017 at Hospital Británico de Buenos Aires. The incidence of AKI was defined as per the Kidney Disease: Improving Global Outcomescriteria, based on pre- and post-operative bloodcreatinine levels and urine output.
Results
A total of 125 patients were included. Of them, 35 % developed AKI. The analysis of riskfactors showed a statistically significant differencefor the administration of vancomycin and thiazidediuretics, red blood cell transfusion requirement, extracorporeal circulation pump time, clamp time, maximal intraoperative lactate level, minimumtemperature, and delayed chest closure. In relationto the parameters of the post-operative course, weobserved a longer hospital stay, higher inotropicrequirement, more days of mechanical ventilation, bleeding, and neurological complications.
Conclusion
In this study, the incidence of AKI was 35 %. Modifiable and non-modifiableassociated risk factors were defined and a greaterrate of complications was observed in patientswho developed AKI.
Key words Acute kidney injury; Cardiovascular surgery; Pediatrics
INTRODUCTION
Acute kidney injury (AKI) has been described as a commoncomplication of cardiac surgery inpediatric patients, whose impact onmorbidity and mortality has beendocumented. Its incidence has beendescribed to be approximately 40 %in this patient group.1
AKI has been associated with more days of mechanical ventilation, ahigher inotropic requirement, a longerlength of stay, and mortality, evenin patients with small variations increatinine levels.2,4
Delayed growth, hypertension, and chronic kidney failure have beendescribed in the long term.5,6
The risk factors include high preoperative blood creatinine levels, age younger than 1 year, prolongedextracorporeal circulation time, higherinotrope requirements, and low postoperative cardiac output.1,3,7
Likewise, an association has been described with the greater surgerycomplexity,8 which is reflected on therisk adjustment for congenital heartsurgery (RACHS-1) category.9,10
There are different definitions of AKI, which have graduallyevolved seeking for a more accurateassessment. For example, the Risk, Injury, Failure, Loss, and End-stage (RIFLE) criteria, their pediatricadaptation (p RIFLE), and the Acute Kidney Injury Network (AKIN) classification. More recently, in 2012, the Kidney Disease: Improving Global Outcomes (KDIGO) group developednew criteria in an attempt to reconcilethe differences among the previous definitions,2,11,12 and they have been validated in both children and adults.13,15
The pathophysiology of AKI in these patients involves multiple mechanisms: a low cardiacoutput, the release of pro inflammatory cytokines, the ischemia-reperfusion phenomenon, bloodmechanical trauma, oxidative stress, andnephrotoxins.5,15
Identifying the risk factors would allow to develop strategies aimed at reducing morbidityand mortality. Therefore, the objective of thisstudy was to estimate the incidence of AKI inpatients who underwent cardiovascular surgeryand to determine the associated risk factors andthe impact of AKI on the parameters of the postoperative course.
POPULATION AND METHODS
Design
This was a retrospective, observational cohort study of pediatric patients who underwentcardiovascular surgery between January 2015 and December 2017 at Hospital Británico de Buenos Aires, a referral tertiary care facility. Medicalrecords were reviewed to collect the data of thestudy variables to be analyzed.
Eligibility criteria
Inclusion criteria
Age younger than 16 years.
Surgery performed by the hospital's Cardiac Surgery Team between January 2015 and December 2017.
Exclusion criteria
Recorded study variables
Incidence of AKI: As per the KDIGO criteria (see Annex).
Risk factors: Age, sex, weight, gestational age, birth weight, family history, comorbidities, type of heart disease, priorsurgery, nephrotoxic drugs, inotropic use, RACHS-1 category, hypotension, transfusions, intravenous fluids, hyperglycemia, infections, arrhythmia, post-operative catheterization, extracorporeal circulation pump time, clamp time, delayed chest closure, maximallactate level, minimum temperature, graft orprosthetic material use.
AKI assessment: Creatinine levels, urea levels, and urine output (pre-operative, postoperative, at the time of AKI diagnosis, andworst level achieved).
Parameters of post-operative course: Length of stay in days, days of mechanicalventilation (MV), inotropes, renal functionreplacement therapy, cardiorespiratoryarrest, extracorporeal membrane oxygenation (ECMO) requirement, multiple organ failure, and death. In addition, a combined event wasdefined, which included the occurrence ofdeath and neurological, liver, infectious orbleeding post-operative complications.
Ethical aspects
This study was approved by the hospital's Institutional Review Board in accordance withthe Declaration of Helsinki. Due to the study'sretrospective nature, we requested the waiver ofinformed consent.
Statistical analysis
Continuous variables were described as mean and standard deviation (SD) for thosewith a normal distribution or as median andinterquartile range (IQR) for those with anabnormal distribution. Categorical data wereexpressed as frequency and percentage. Theassumption of normality was assessed using the Shapiro-Wilk test. Continuous data were assessedusing the t test in the case of normal distributionor the Mann-Whitney U test in case of abnormaldistribution. Categorical data were compared with the χ2 test or Fisher's exact test, as applicable.
The association between potential risk factors and the occurrence of AKI was assessed usinga multivariate logistic regression model, whichincluded all variables with a p value < 0.1 inthe univariate analysis (besides age, sex, andweight). The final model was selected by makingall possible combinations of predictor variablesincluded in the initial model and leaving thosethat showed a statistically significant associationand the best predictive power (as per the Akaikeinformation criterion). If predictor variables werecontinuous, receiver operating characteristic (ROC) curves were developed and the best cutoff point was identified using the Youden index. Regression analyses were then repeated usingthe dichotomized variables. For each predictorvariable, the odds ratio (OR) and its corresponding95 % confidence interval (CI) were calculated.
To assess the association between AKI and post-operative outcomes, multivariate regressionmodels were used (a logistic regression for thecombined event and a Poisson regression forthe length of stay in days), and the developmentof AKI was used as a predictor variable, withadjustments for other confounding factors. Thevariables for these models were selected in thesame way as described above for the occurrenceof AKI, except that AKI was forced into themodel in both cases. The multivariate associationbetween length of stay in days and AKI in the Poisson regression analysis was described as anincidence rate ratio (IRR) and its corresponding95 % CI.
All analyses were two-tailed, and a p value < 0.05 was considered statistically significant. The sample size was determined by conveniencebecause, due to logistical reasons, it was possibleto include patients between the dates mentionedabove. Analyses were done using the Graph Pad Prism 8.0.1® and the R 3.5.1 software programs.
RESULTS
Characteristics of the population under study
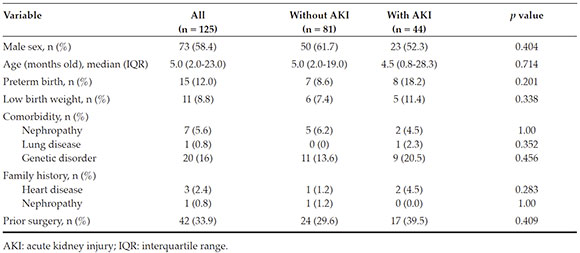
During the study period, 145 patients underwent surgery; of them, 20 met the exclusioncriteria, so a total of 125 patients were eligibleand included in the study group. The incidenceof post-operative AKI was 35 % (n = 44). In thisgroup, defined as "AKI patients," 70.5 % (n = 31),22.7 % (n = 10), and 6.8 % (n = 3) met the criteriafor categories 1, 2, and 3 of AKI, respectively.Table 1 shows the characteristics of the populationunder study.
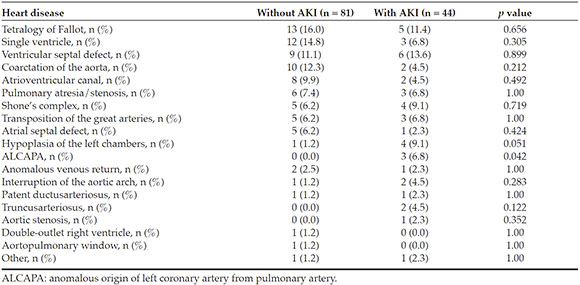
No statistically significant differences were observed in relation to the type of heartdisease, except in the case of anomalous originof left coronary artery from pulmonary artery (ALCAPA), which was more frequent in AKI patients (Table 2).
Risk factors for acute kidney injury
Table 3 shows the distribution of risk factors for AKI. Variables associated with thedevelopment of AKI in the univariate analysiswere the administration of vancomycin (p = 0.012) and the use of thiazides (p = 0.015). However, no association was observed between AKI andinfections, thrombosis, arrhythmia, greatersurgical complexity as per the RACHS-1 or postoperative catheterization as risk factors.
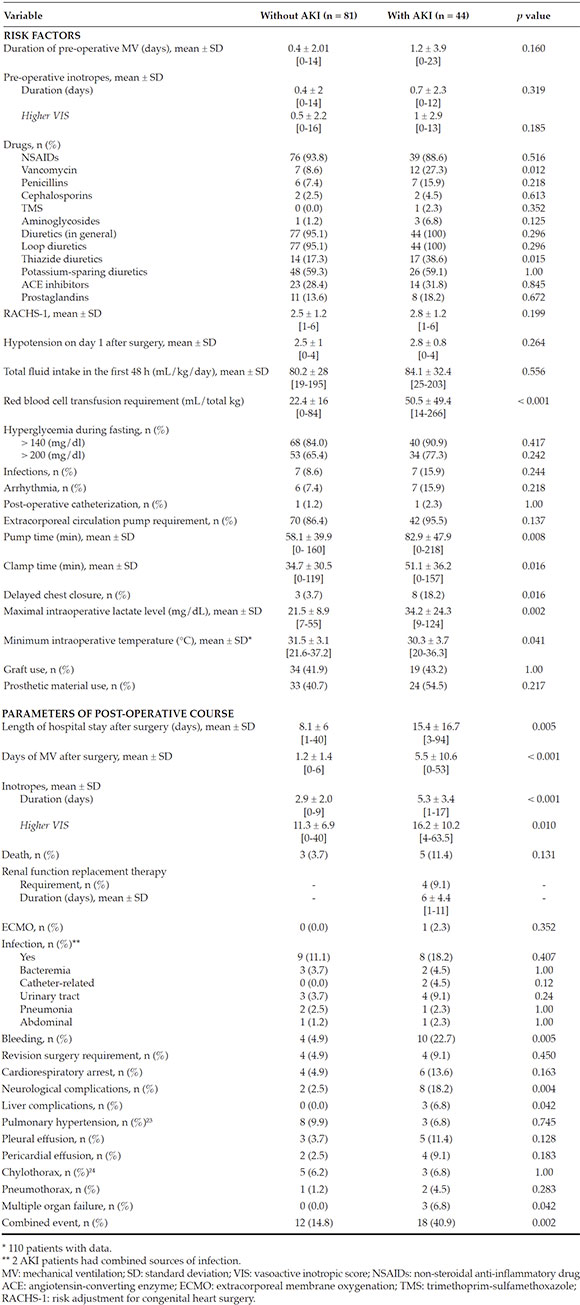
Table 3 Pre-, intra-, and post-operative variables analyzed as potential risk factors and parameters of post-operative course
AKI patients showed a non-significant tendency towards a higher frequency ofhyperglycemia. When the hypoperfusion andtissue hypoxia variables were analyzed aspotential event triggers, AKI patients wereobserved to have a significantly higherrequirement for red blood cell transfusions (p< 0.001). However, no significant differenceswere observed for pre-operative inotropicrequirement as per the vasoactive inotropic score (VIS),16,17 the hypotension score (cardiovascularsection of the Pediatric Sequential Organ Failure Assessment [ p SOFA])1,18 or the fluid intake in thefirst 48 h, as an indirect measure of low cardiacoutput.
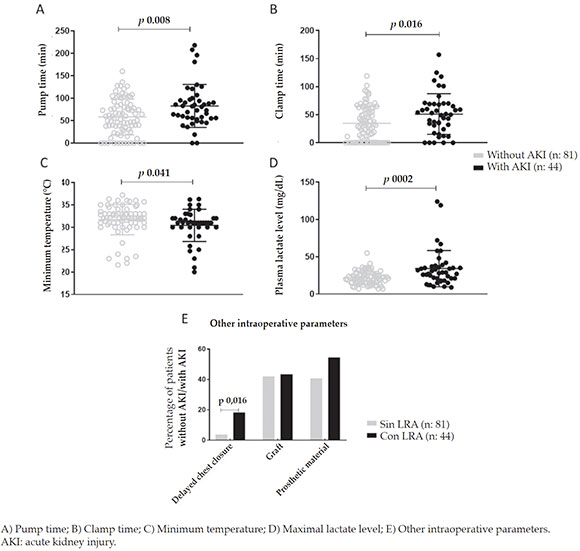
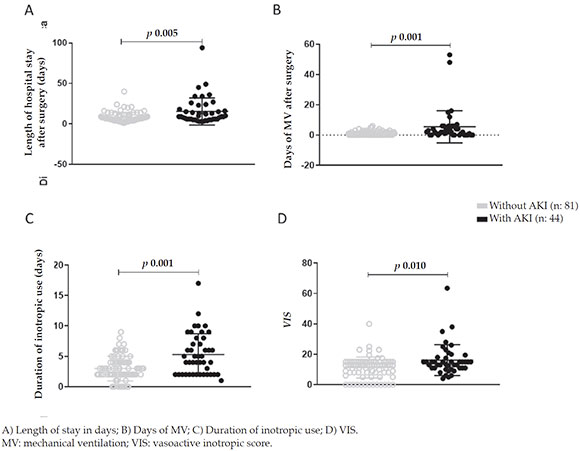
A statistically significant difference was noted in terms of the intraoperative risk factors, such asextracorporeal circulation pump time (p = 0.008), clamp time (p = 0.016), delayed chest closure (p = 0.016), maximal lactate level (p = 0.002), and minimum temperature (p = 0.041) (Figure 1).
The multivariate logistic regression analyses identified red blood cell transfusion and maximallactate level as independent variables. For the firstone, a cut-off value of 35 m L/kg or higher wasidentified (OR: 3.26; 95 % CI: 1.31-8.21; p = 0.011), whereas for the second, the cut-off point was setat 26.5 mg/d L or higher (OR: 3.11; 95 % CI: 1.287.61; p = 0.012).
Parameters of post-operative course
Table 3 shows the analysis of these parameters. A statistically significant increase in the lengthof stay in days and in the days of MV wasobserved in AKI patients (p = 0.005 and p < 0.001, respectively) (Figure 2. A-B). Likewise, a longerduration of inotropic use (p < 0.001) and a higherinotropic use as per the VIS (p = 0.010) wereobserved (Figure 2. C-D).
The development of AKI was associated with a higher risk for bleeding (p = 0.005) andneurological complications (p = 0.004). In total,11.4 % (n = 5) of AKI patients died, comparedto 3.7 % (n = 3) of those who did not develop AKI (p = 0.131). All patients with multiple organfailure (n = 3) had AKI, which accounted for 6.8 %of them. The only patient who was on ECMO haddeveloped stage 3 kidney injury with peritonealdialysis requirement. Out of all AKI patients,9.1 % required peritoneal dialysis. The combined event occurred in 12 patients (14.8 %) without AKI and in 18 (40.9 %) with AKI (p = 0.002).
The association between the length of stay in days and the development of AKI was no longerstatistically significant after the adjustment forconfounding factors (IRR: 1.05; 95 % CI: 0.911,20; p = 0.505). Something similar was observedwith the combined event: the association betweenit and AKI was no longer significant after theadjustment for confounding variables (OR: 1.88;95 % CI: 0.46-7.62; p = 0.370).
Parameters for the measurement of acute kidney injury
The patients who developed AKI had, in the immediate post-operative period, a higher bloodcreatinine level (0.42 ± 0.2 versus 0.36 ± 0.1 mg/d L; p = 0.020) and a lower urine output (1.6 ± 1.3m L/kg/h versus 2.5 ± 1.4 m L/kg/h; p < 0.001)( Figure 3. A-B ). In relation to the time of AKI onset, 57 % of patients developed it in the firstday after the surgery; 29 %, on the second day; and the rest, in the subsequent days; the latestcase occurred 27 days after the surgery. In relationto the duration in hours, most cases resolvedwithin 24 h (65 %) ( Figure 3. C-D ).
The association between the AKI stage and the RACHS-1 categories was not observed to bestatistically significant, but there was a relevantproportional increase in the AKI stage based onthe RACHS-1 category.
DISCUSSION
AKI is one of the most common complications in pediatric patients who undergo surgery for congenital heart disease. The incidence of AKI in our patients, as per the KDIGO criteria, was 35 %, which is slightly below what has been described in the bibliography. 1,3 -5,19 Such difference is attributed to the fact that most of
the published case reports defined AKI as per the p RIFLE criteria (before the validation of the KDIGO criteria in pediatrics), which had a greatersensitivity and a lower specificity,19,20 especiallyin the first stages of risk. Most cases developed inthe first day after the surgery; the criteria for stage1 AKI were met; and AKI was resolved in the first24 h, similar to what has been described to date.1,3
In addition, a greater incidence of AKI was not observed in patients younger than1 month, unlike what has been published inthe bibliography.3,5 Such discrepancy maybe due to the fact that the KDIGO criteriameasure a variation in blood creatinine levels (instead of creatinine clearance), and it hasbeen described that this population has highblood creatinine levels during the first days oflife provided by the mother, so the absolutebaseline value may be higher.19 This would makeit harder to detect a percent increase to meet thediagnostic criterion. However, the KDIGO criteriacurrently recommended to define AKI have anintermediate sensitivity compared to the previoustwo instruments12,19,20 and are easily implementedbecause of the variables used (height is notinvariably required to estimate the glomerularfiltration rate, which is usually underestimatedin medical records).
Among the studied risk factors, no differences were observed in terms of the incidence of AKI as per the RACHS-1 category, but there weredifferences between this and the AKI stage in thepatients who developed it. It would appear thenthat, although surgery for congenital heart diseaseis a risk factor itself for the development of AKI and such complication may have a multifactorialorigin, the greater surgical complexity wouldfavor the development of higher AKI stages. Among the studied nephrotoxic drugs, anassociation was observed between AKI andthe use of vancomycin and thiazide diuretics. For this reason, it may be concluded that, aswell as in the general pediatric population, inthese patients, strategies for the rational use ofantibiotic, diuretics and other drugs should berecommended; because, even though these arecomplex patients with prolonged hospitalizationsand risk for infection, it is important to considerthe greater impact that drug nephrotoxicity has onthem. Consistent with prior publications, a greaterincidence of AKI in patients who had higherhyperglycemia levels has been demonstrated;21,22 however, such association was not statisticallysignificant and, based on the studies reviewed todate, no recommendations have been found inrelation to a strict hyperglycemia control.7,22
Considering that a pathophysiological hypothesis suggests that a low cardiac output and tissue hypoxia may trigger AKI,1,19 variables for the indirect measurement of these two factorshave been analyzed. An association was observedbetween a greater red blood cell transfusionrequirement and the incidence of AKI, but notbetween AKI and a higher inotropic requirementin the pre-operative period, hypotension score ora higher total fluid intake. On the basis of this, theimpact of tissue hypoxia (as indirectly measuredby transfusion requirement) would be greaterthan that of a low cardiac output. In addition, and considering the intra-operative variables, ithas been observed that the incidence of AKI washigher with a longer pump time, clamp time andhigher plasma lactate levels and with a lowertemperature during surgery. These findings weresimilar to those reported in the bibliography.1,3,14,19
Finally, the analysis of the impact of AKI on morbidity and mortality showed that AKI patients had a greater rate of multiple organfailure and death. Likewise, in this group, alonger length of stay after the surgery, moredays of MV, a higher inotropic requirement, and a greater incidence of bleeding, liver andneurological complications were detected.
CONCLUSIONS
The incidence of AKI in pediatric patients who underwent cardiovascular surgery was 35 % in thisstudy.
The analysis of the assessed risk factors showed a statistically significant differencefor the administration of vancomycin andthiazide diuretics, red blood cell transfusionrequirement, extracorporeal circulation time, clamp time, maximal intraoperative lactatelevel, minimum temperature, and delayed chestclosure. In relation to the parameters of the post-operative course, we detected a statistically significant longer hospital stay, higher inotropicrequirement, more days of mechanical ventilation, bleeding, and neurological complications.
REFERENCES
1. Li S, Krawczeski CD, Zappitelli M, Devarajan P, et al. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: a prospective multicenter study. Crit Care Med. 2011;39(6):1493-9. [ Links ]
2. Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 2017;376(1):11-20. [ Links ]
3. Aydin SI, Seiden HS, Blaufox AD, Parnell VA, et al. Acute Kidney Injury After Surgery for Congenital Heart Disease. Ann Thorac Surg. 2012;94(5):1589-95. [ Links ]
4. Dos Santos El, Halal MG, Carvalho PR. Acute kidney injuryaccording to pediatric RIFLE criteria is associated with negative outcomes after heart surgery in children. Pediatr Nephrol. 2013;28(8):1307-14. [ Links ]
5. Jefferies JL, Devarajan P. Early detection of Acute Kidney Injury after pediatric cardiac surgery. Prog Pediatr Cardiol. 2016;41:9-16. [ Links ]
6. Madsen NL, Goldstein SL, Frolsev T, Christiansen CF, et al. Cardiac Surgery in patients with congenital heart disease isassociated with acute kidney injury and the risk of chronickidney disease. Kidney Int. 2017;92(3):751-6. [ Links ]
7. Blinder JJ, Asaro LA, Wypij D, Selewski DT, et al. Acute Kidney Injury After Pediatric Cardiac Surgery: A secondary Analysis of the Safe Pediatric Euglycemia After Cardiac Surgery Trial. Pediatr Crit Care Med. 2017;18(7):638-46. [ Links ]
8. Kumar TK, Allen Ccp J, Spentzas T, Berrios Ccp L, et al. Acute Kidney Injury Following Cardiac Surgery in Neonatesand Young Infants: Experience of a Single Center Using Novel Perioperative Strategies. World J Pediatr Congenit Heart Surg. 2016;7(4):460-6. [ Links ]
9. Ithuralde M, Ferrante D, Seara C, Ithuralde A, et al. Análisis de la mortalidad y distribución de procedimientos de cirugía de cardiopatías congénitas utilizando el método de ajuste de riesgo RACHS-1. Rev Argent Cardiol. 2007;75(3):179-84. [ Links ]
10. Jacobs JP, Jacobs ML, Lacour-Gayet FG, Jenkins KJ, et al. Stratification of Complexity Improves the Utility and Accuracy of Outcomes Analysis in a Multi-Institutional Congenital Heart Surgery Database: Application of the Risk Adjustment in Congenital Heart Surgery (RACHS-1) and Aristotle Systems in the Society of Thoracic Surgeons (STS) Congenital Heart Surgery Database. Pediatr Cardiol. 2009;30(8):1117-30. [ Links ]
11. Kellum JA, Lameire N, Aspelin P, Barsoum RS, et al. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2(1):1-138. [ Links ]
12. Sutherland SM, Byrnes JJ, Kothari M, Longhurst CA, et al. AKI in Hospitalized Children: comparing the p RIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol. 2015;10(4):554-61. [ Links ]
13. Selewski DT, Cornell TT, Heung M, Troost JP, et al. Validation of the KDIGO acute kidney injury criteria in apediatric critical care population. Intensive Care Med. 2014;40(10):1481-8. [ Links ]
14. Gist KM, Kwiatkowski DM, Cooper DS. Acute kidneyinjury in congenital heart disease. Curr Opin Cardiol. 2017;32(1):101-7. [ Links ]
15. Toda Y, Sugimoto K. AKI after pediatric cardiac surgery forcongenital heart diseases-recent developments in diagnosticcriteria and early diagnosis by biomarkers. J Intensive Care. 2017;5:49. [ Links ]
16. Gaies MG, Jeffries HE, Niebler RA, Pasquali SK, et al. Vasoactive-Inotropic Score is associated with outcome after infant cardiac surgery: an analysis from the Pediatric Cardiac Critical Care Consortium and Virtual PICU System Registries. Pediatr Crit Care Med. 2014;15(6):529-37. [ Links ]
17. Davidson J, Tong S, Hancock H, Hauck A, et al. Prospective validation of the vasoactive-inotropic score and correlation to short term outcomes in neonates and infants after cardiothoracic surgery. Intensive Care Med. 2012;38(7):1184-90. [ Links ]
18. Matics T, Sanchez-Pinto L. Adaptation and Validation ofa Pediatric Sequential Organ Failure Assessment Scoreand Evaluation of the Sepsis-3 Definitions in Critically illchildren. JAMA Pediatr. 2017;171(10):e172352. [ Links ]
19. Park SK, Hur M, Kim E, Kim WH, et al. Risk factors for Acute Kidney Injury after Congenital Cardiac Surgery in Infants and Children: A Retrospective Observational Study. PLo S One. 2016;11(11):e0166328. [ Links ]
20. Lex D, Toth R, Cserép Z, Alexander SI, et al. A comparisonof the systems for the identification of postoperative acutekidney injury in pediatric cardiac patients. Ann Thorac Surg. 2014;97(1):202-10. [ Links ]
21. Preissig CM, Rigby MR, Maher KO. Glycemic control forpostoperative pediatric cardiac patients. Pediatr Cardiol. 2009;30(8):1098-104. [ Links ]
22. Agus MSD, Wypij D, Hirshberg EL, Srinivasan V, et al. Tight Glycemic Control in Critically Ill Children. N Engl J Med. 2017;376(8):729-41. [ Links ]
23. Abman SH, Hansmann G, Archer SL, Ivy DD, et al. Pediatric Pulmonary Hypertension: Guidelines fromthe American Heart Association and American Thoracic Society. Circulation. 2015;132(21):2037-99. [ Links ]
24. Soto-Martinez M, Massie J. Chylothorax: diagnosis andmanagement in children. Pediatr Respir Rev. 2009;10(4):199-207. [ Links ]
Received: August 8, 2018; Accepted: April 29, 2019











 texto em
texto em