Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista argentina de microbiología
Print version ISSN 0325-7541On-line version ISSN 1851-7617
Rev. argent. microbiol. vol.39 no.3 Ciudad Autónoma de Buenos Aires July/Sept. 2007
Tuberculosis transmission of predominant genotypes of Mycobacterium tuberculosis in Northern suburbs of Buenos Aires city region
N. Morcillo1*, M. Zumarraga2, B. Imperiale1, B. Di Giulio3, C. Chirico4, A. Kuriger4, A. Alito2, K. Kremer5, A. Cataldi2
1 Mycobacteria Reference Laboratory, Tuberculosis Control Program of Buenos Aires Province, Dr. Cetrángolo Hospital, Vicente López, Buenos Aires, Argentina;
2 National Institute of Agricultural Technology, Biotechnology Laboratory (INTA/CICV), Castelar, Buenos Aires, Argentina;
3 P. de Cordero Hospital, San Fernando, Argentina;
4 Regional Tuberculosis Control Program, Vicente López, Buenos Aires, Argentina;
5 National Institute of Public Health and the Environment (RIVM), Bilthoven, The Netherlands.
*Correspondence. E-mail: nora_morcillo@yahoo.com.ar
ABSTRACT
In 2003, the incidence of tuberculosis in Argentina showed an increase compared to 2002. The severe national crisis at the end of the 90s has probably strongly contributed to this situation. The goal of this work was to estimate the extent of the spread of the most predominant Mycobacterium tuberculosis strains and to assess the spread of predominant M. tuberculosis clusters as determined by spoligotyping and IS6110 RFLP. The study involved 590 pulmonary, smear-positive TB cases receiving medical attention at health centers and hospitals in Northern Buenos Aires (NBA) suburbs, from October 2001 to December 2002. From a total of 208 clinical isolates belonging to 6 major clusters, 63 (30.2%) isolates had identical spoligotyping and IS6110 RFLP pattern. Only 22.2% were shown to have epidemiological connections with another member of their respective cluster. In these major clusters, 30.2% of the 208 TB cases studied by both molecular techniques and contact tracing could be convincingly attributable to a recently acquired infection. This knowledge may be useful to assess the clonal distribution of predominant M. tuberculosis clusters in Argentina, which may make an impact on TB control strategies.
Key words: Tuberculosis; Clusters; Predominant clones; Genotyping
RESUMEN
Transmisión de la tuberculosis por genotipos predominantes de Mycobacterium tuberculosis en la región Gran Buenos Aires Norte. La incidencia de la tuberculosis en Argentina mostró en 2003 un incremento en comparación con 2002. La grave crisis nacional a fines de los 90 ha probablemente contribuido en gran medida a esta situación. El objetivo del presente trabajo fue determinar la diversidad genética de aislamientos de Mycobacterium tuberculosis y el grado de dispersión de algunas cepas mayoritarias genéticamente relacionadas. El estudio involucró 590 aislamientos clínicos provenientes de muestras respiratorias con examen directo positivo, de pacientes atendidos en los hospitales y centros de salud que conforman la región Gran Buenos Aires Norte (NBA), de octubre de 2001 a diciembre de 2002. De 208 aislamientos que se encontraron en los 6 mayores clusters, 63 (30,2%) tenían patrones idénticos de spoligotyping y de IS6110 RFLP. En el 22,2% de los casos fue posible verificar la conexión epidemiológica con otro miembro del respectivo cluster. Concluimos que el 30,2% de estos agrupamientos principales pueden ser atribuidos a una infección reciente. Estos resultados pueden ser útiles para determinar la distribución clonal de los grupos predominantes de M. tuberculosis en Argentina, lo que puede impactar en las estrategias de control de la tuberculosis.
Palabras clave: Tuberculosis; Agrupamientos; Clones predominantes; Genotipificación
INTRODUCTION
Tuberculosis (TB) incidence in Argentina has remained almost stable for the last 20 years. However, in 2003 the incidence was 32.0 per 100,000 inhabitants, showing a 2.5% increase in comparison with the year 2002 (30.5 per 100, 000) (16). Furthermore, it varies significantly per area and high incidences are recorded in Buenos Aires Province (36.4 per 100,000), with the highest figures in the northern suburbs of Buenos Aires City (NBA, 46.0 per 100,000). During the year 2005, the incidence rate was 30.4 per 100,000 cases in Argentina, 33.2 per 100,000 in Buenos Aires Province and 43.2 per 100,000 in NBA. In this area, with more than 3.5 million inhabitants and 14 municipalities, a total of 1,480 new TB cases were reported in 2002 (16).
Molecular techniques for differentiation of Mycobacterium tuberculosis strains, such as restriction fragment length polymorphism (RFLP) and spoligotyping have been used for more than a decade to study the epidemiology of TB (7, 8, 13, 18, 22, 28). RFLP analysis with IS6110 as a probe, was used in several epidemiological investigations, providing compelling evidence of institutional transmission (2, 3, 5, 23). Furthermore, this method was used to identify cases of unsuspected transmission, for tracking laboratory cross-contamination, and for discriminating exogenous versus endogenous infection (3, 4, 6, 20, 25). Besides, it is assumed that two or more clinical M. tuberculosis isolates sharing identical IS6110 RFLP patterns (defined as cluster) indicate in most cases both person-toperson transmission or transmission from a similar source, as well as a recently acquired infection (24, 29).
Spoligotyping, is also useful to differentiate the species belonging to the M. tuberculosis complex (12, 28). The ability of spoligotyping to differentiate M. tuberculosis strains is lower than that of IS6110 RFLP, but being a rapid and cheaper technique, it can be used to screen clinical isolates from a larger TB population (9, 11). Isolates with identical spoligotype patterns should be subjected to additional typing with the most discriminatory IS6110 RFLP to study tuberculosis transmission.
In a first approach, this study investigates the genetic diversity of predominant clinical isolates of M. tuberculosis from NBA patients by using spoligotyping and IS6110 RFLP. In addition, the aims were also to estimate the extent of spread of these predominant M. tuberculosis strains which form large TB clusters (larger than 10 patients).
MATERIALS AND METHODS
Population study
This study was carried out from October 2001 to December 2002, involving 622 cases from health institutions in the area NBA (21 health centres and 15 hospitals), which were requested to submit smears and/or cultures, as well as clinical and bacteriological data from the identified TB cases, to Dr. Cetrángolo Hospital. The information included location of the disease, previous treatment history, HIV infection, smears, cultures and drug susceptibility testing results. Epidemiological data obtained from the medical records were also included in the database: age; gender; residence; the fact of being a health care worker; household or occasional contact with a recent or past TB case. Contacts were identified by social workers interviewing the TB patient and more frequent contacts, followed by clinical radiological and if needed, bacteriological examination.
A total of 622 respiratory clinical specimens were processed by smears for acid-fast bacilli detection and cultures on Lowenstein-Jensen (LJ) medium and MGIT960 (BD, Buenos Aires, Argentina) (14, 31). The LCx M. tuberculosis Assay (Abbott Laboratories, USA) was used to identify the M. tuberculosis complex isolates.
The susceptibility to isoniazid (INH); streptomycin; rifampin (RMP) and ethambutol was investigated by the classical proportion method on LJ medium and MGIT960 (15). RMP-resistance was also explored by analysis of the rpoB gene of M. tuberculosis by using a reverse line blot hybridization assay following the previously published protocol (21).
Spoligotyping was carried out on all the isolates. Considering unlikely that isolates with different spoligopattern belong to the same cluster, IS6110 RFLP typing was performed on M. tuberculosis isolates that belonged to an spoligotyping cluster. From previous studies and patients without any verifiable epidemiological relationship among them, we found different IS6110 RFLP patterns from isolates belonging to a small spoligotyping cluster (2 or 3 isolates) (data not published). Therefore, we decided to perform this last technique on clusters comprising at least 10 or more isolates. To estimate the highly predominating M. tuberculosis clones in the community, which could be responsible for recent transmission, IS6110 RFLP typing was carried out on all M. tuberculosis isolates that belonged to spoligotype clusters comprising 10 or more isolates. Both techniques were performed by following the standard protocols previously published (12, 27). Genetic patterns were analysed by the Gel Compar software (version 4.1, Applied Maths, St- Martens-Latem, Belgium). The IS6110 RFLP patterns were compared by using the Dice coefficient for calculating the similarities between the patterns, and by tolerating 1% position variation. Patients with genetically related isolates were considered clustered. Patients whose isolate patterns exactly matched were assigned a single cluster designation. For spoligotyping, a cluster was also defined as two or more strains with identical patterns.
The reference strain H37Rv was used as reference standard for drug-susceptibility testing as well as the molecular techniques used.
Statistical methods
The MedCalc software (version 7.3.0.0, MedCalc®, Mariakerke, Belgium) was used to calculate summary statistics of the included variables. The significance level was established at 5.0 %. Univariate associations between demographic and clinical variables and the dependent variable (clustered isolates) were examined by frequency tables and the Chi square test. Associations were also examined by multiple logistic regression analysis.
RESULTS
Data from 622 out of 803 (77.5%) respiratory TB cases notified to the NBA TBCP during this 14 month- period were collected. Neither epidemiological information nor clinical specimens from the remaining 181 cases were sent to the laboratory to be processed. Due to contaminated cultures 32 out of 622 (5.1%) cases were also excluded; 590 patients that represented 73.5% (590/803) of the bacteriologically proved individual cases were finally included in the study. The median age of the patients was 35.1 years (95% CI: 33.8-36.2. range: 1-78); 230 (39.0%) women and 360 (61.0%) men (P: 0.0001); 125 (21.2%) cases were co-infected with HIV; 115 (19.5%) cases had previously received TB treatment; 7 (1.2%) were health care workers, and in 48 (8.1%) cases, epidemiological links were demonstrated. The same demographic characteristics were investigated in the 213 respiratory TB cases not included in this study, which were notified to the NBA TBCP. This investigation demonstrated that the patient characteristics of this excluded group did not differ significantly from the 590 patients included in this study (data not shown).
Drug susceptibility testing showed that 434 out of 590 (73.6%) M. tuberculosis complex isolates were susceptible to first-line anti-tuberculosis drugs (MTS); 75 (12.7%) isolates were multidrug-resistant (MDR; resistant to at least INH and RMP), and 81 (13.7%) strains were resist ant to one or more drugs but not to isoniazid plus rifampicin (DR) (Table 1). This high occurrence of MDR strains was specific to this region and during the period studied, whereas the overall incidence in the country was lower. Besides, the MDR figures were obtained by including all the prevalent cases occurring in the NBA region. Furthermore, it does not represent the MDR incidence rate in NBA but the prevalence at that moment. Most of these cases had received previous anti-TB treatment.
Table 1. Unique and clustered spoligotype patterns, drug resistance profile of the isolates and the epidemiologic and clinical characteristics of the studied patients.

All M. tuberculosis complex isolates were also subjected to analysis of the rpoB gene by the reverse line blot hybridisation assay. The assay correctly identified all RMP-resistant isolates and showed that three mutations conferring resistance to RMP predominated in the NBA population. These point mutations in the rpoB gene were: TCG Ser531Leu 74.7% (56/75); CAC His526 Asp 16.0% (12/75); and TGG Ser531Trp: 5.3% (4/75).
All 590 isolates were successfully subjected to spoligotyping as a screening technique, yielding 249 different spoligotype patterns; 190 out of 590 (32.2%) isolates showed a unique spoligotype pattern, whereas 67.8% (400/590) were grouped into 59 clusters with an average of 6.8 isolates (range 2-52) (Table 1). Table 1 shows the correlation between patient characteristics and spoligotype results. No statistical differences were found between the patient characteristics whose isolates had unique patterns and those of patients clustered by spoligotyping, except that the comparison between children under 15 and adults aged 15-35 years neared statistical significance. Children aged under 15 years more frequently belonged to a spoligotype cluster than adults aged between 15 to 35 years: 22/26 (84.6%) versus 145/230 (63.1%) (P: 0.0002). Similarly, 250 (69.4%) men and 150 (65.2%) women were clustered (P: 0.3299). HIV-positive and HIV-negative cases were equally frequently found in spoligotype clusters 84 (67.2%) and 316 (68.0%), respectively (P: 0.9610), as cases were with (79; 68.7%) and without previous anti-TB treatment (321; 67.6%) (P: 0.9577). Table 1 shows that 58 (72.2%) DR, 287 (66.1%) MTS and 55 (74.3%) MDR organisms had clustered spoligotype patterns.
The isolates of the six largest spoligotype clusters, containing 13 to 52 isolates and involving 35.3% (208/ 590) of the strains, were subjected to IS6110 RFLP typing to investigate the extent to which large chains of transmission contribute to the tuberculosis problem in NBA. All six spoligotype clusters were sub-divided. Table 2 shows the heterogeneity among the IS6110 RFLP paterns of the isolates clustered by spoligotyping, expressed as the percentage of similarity found among the RFLP patterns. Sixty-three out of 208 (30.2%) isolates had identical genetic profiles by both spoligotyping and IS6110 RFLP typing, representing six clusters of six to 16 isolates. A total of 115 (55.3%) out of these 208 isolates grouped in a spoligotype cluster, which contained isolates having more than 95% similarity in their RFLP patterns. Furthermore, 49.0% (102/208) of the strains identified by spoligotyping as belonging to a cluster shared between 95% and 80% similarity among their IS6110 RFLP banding patterns. Although the spoligotype pattern was identical, 20.2% (42/208) of the strains showed a similarity of only 76% to 62% among their IS6110 RFLP patterns (Table 2).
Table 2. Percentage of similarity found among the IS6110 RFLP patterns from strains clustered by spoligotyping.

Figure 1 displays the prevalent IS6110 RFLP patterns related to the spoligotype clusters A, B, C, D, E and F. Two out of 7 isolates from health care workers were found in clusters: one of them belonged to the major cluster A and 4 were identified as MDR strains. One of these latter mentioned strains belonged to a cluster, which had caused the first MDR-TB nosocomial outbreak in our hospital from 1992 to 1994 (20).
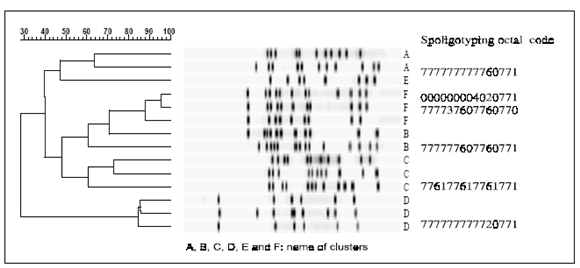
Figure 1. IS6110 RFLP patterns of the six predominant spoligotype clusters.
Nosocomial and familiar transmission were verified among patients from cluster E and F respectively, whose isolates shared the same spoligotyping, IS6110 RFLP and drug-resistance patterns. Epidemiological connections among members of their respective cluster were found in 22.2% (14/63) of the clustered patients.
Nineteen out of 590 (3.2%) strains showed a spoligotype pattern characteristic of the Haarlem genotype (10, 18). This strain was previously identified as the causal agent that had provoked MDR-TB nosocomial outbreaks in Argentina (20, 23). From 6 patients, (1.0%) the obtained mycobacteria had spoligotyping and IS6110 RFLP patterns compatible with those of the Beijing family (17, 18). These organisms were isolated from a community living in a small district of our region.
Risk factors associated to recent transmission, expressed as strains clustered by IS6110 RFLP, were explored by logistic regression analysis. The results showed that an epidemiological connection (household or circumstantial contact with a confirmed TB case) was associated with clustering (O.R. 2.26, 95% CI: 0.96- 5.32). When adjusted by gender, the association was even stronger in women than in men (O. R. 2.63, 95% CI: 0.79-8.82). HIV infection, MDR, and previous treatment showed a slight and similar association with clustering, but these associations were not significant; HIV, O. R. 1.06, 95% CI: 0.50-2.26; MDR-TB, O.R. 1.21, 95% CI: 0.55-2.67; and previously treated cases, O.R. 1.24, 95% CI: 0.57-2.69.
DISCUSSION
This study has been conducted in a big area in the Northern suburbs of Buenos Aires City, attempting to assess the proportion of cases probably attributable to a recent transmission of M. tuberculosis predominant strains and the risk factors related to it.
One of the main goals was to provide information about predominant clones of M. tuberculosis actively causing TB disease in an Argentine community living in a welldelimited geographical area, as well as to estimate the amount of cases probably due to either reactivation of a latent infection or the acquisition of a new strain from an exogenous contagious source.
In this study, 35.2% (208/590) of the cases investigated by spoligotyping were grouped into 6 major clusters and from them, 30.2% (63/208) of the isolates analyzed by spoligotyping and IS6110 RFLP, were clustered by both methods. Besides, 22.2% (14/63) had verifiable epidemiologic connections with another member of their respective cluster.
From 6 patients (1.0%), the obtained mycobacteria had spoligotyping and IS6110 RFLP patterns compatible with those of the Beijing family (17). These organisms were isolated from a community living in a small district of our region.
Clustering was more frequently associated to children and young people. Considering that the bacteriological diagnosis of TB in children is scarcely reached, the proved disease occurrence in them is an indicator of ongoing M. tuberculosis transmission in the community (2, 19). During this study period, isolates with identical spoligotyping and IS6110 RFLP genetic patterns to that responsible for a MDR-TB nosocomial outbreak in the past and related to that of Haarlem strains (1, 20), were found to have caused active disease in 19 persons. Interestingly, these isolates considered to be a unique strain by genotyping, showed different drug-susceptibility patterns to anti-TB drugs and no epidemiologic links were found among these cases. These findings indicate that these isolates spread within the community.
No specific genetic patterns could be associated either to MDR or HIV cases.
In this study, spoligotyping correctly detected clustered cases confirmed by IS6110 RFLP in the six major spoligotyping clusters. Taking into account that at least 30.2% of the cases in this study might be due to a recent transmission, the lack of specificity decreases the positive predictive value (56.10%) of spoligotyping. In addition to the fact that different spoligopatterns strains can be unlikely related, these findings reinforce the usefulness of spoligotyping as a screening technique.
Several factors might have contributed to the underestimation of the real amount of clustering in this community: losses of cases for technical reasons, the arbitrary selection of clusters with more than 10 isolates, and the assumption that a cluster should have been composed by strains with 100% of identity in their IS6110 RFLP patterns (2). In that regard, several studies carried out on IS6110 evolution, have shown that clonal variants that appear within a relatively short time and whose patterns differ by a few bands may represent recently evolved strains and therefore may be related to an ongoing transmission chain (19, 24, 26, 30).
Acknowledgements: We want to acknowledge Dr. Dick van Soolingen for his kind support to this study and Dr Philip Hopewell for his contribution in the analysis of the results; Marcelo Mazza, Daniel Cardinali, Juan Carlos Donato and Mrs. Zuni Zubov for their technical assistance. Roemmers Foundation Argentina and the European Commission, Project ICA4-CT-2001-10087 partially financed this study. AC is a fellow of the National Research Council of Argentina (CONICET).
REFERENCES
1. Alito A, Morcillo N, Scipioni S, Dolmann A, Romano M, Cataldi A, et al. The IS6110 restriction fragment length polymorphism in particular multidrug-resistant Mycobacterium tuberculosis strains may evolve too fast for reliable use in outbreak investigation. J Clin Microbiol 1999; 37: 788-91.
2. Bennett DE, Onorato IM, Ellis BA, Crawford JT, Schlabe B, Kammerer JS, et al. DNA fingerprinting of Mycobacterium tuberculosis isolates from epidemiologically linked case pairs. Emerg Infect Dis 2002; 8: 1224-9.
3. Caminero J A. Exogenous reinfection with tuberculosis on a European island with a moderate incidence of disease. Am J Respir Crit Care Med 2001; 163: 717-20.
4. Centers for Disease Control and Prevention. Transmission of multidrug-resistant tuberculosis among HIV-infected persons- Florida and New York, 1988-1991. Morb Mortal Wkly Rep 1991; 40: 585-91.
5. Chaves F. Evidence of exogenous reinfection and mixed infection with more than one strain of Mycobacterium tuberculosis among Spanish HIV-coinfected inmates. AIDS 1999; 13: 615-20.
6. Cohn DL, O´Brien R. The use of restriction fragment length polymorphism (RFLP) analysis for epidemiological studies of tuberculosis in developing countries. Int J Tuberc Lung Dis 1998; 2: 16-26.
7. Crawford JT, Braden CR, Schable BA, Onorato IM. National tuberculosis surveillance network: design and methods. Emerg Infec Dis 2002; 8: 1192-6.
8. Dahle UR, Sandven P, Heldal E, Caugant DA. Molecular epidemiology of Mycobacterium tuberculosis in Norway. J Clin Microbiol 2001; 39: 1802-7.
9. De La Salmoniere YOG, Li HM, Torrea G, Bunschoten A, van Embden JDA, Gicquel B. Evaluation of spoligotyping in a study of the transmission of Mycobacterium tuberculosis. J Clin Microbiol 1997; 35: 2210-4.
10. Ellis BA, Crawford JT, Braden CR, McNabb SJN, Moore M, Kammere S et al. Molecular epidemiology of tuberculosis in a sentinel surveillance population. Emerg Infect Dis 2002; 8: 1197-209.
11. Ferdinand S, Sola C, Verdol B, Legrand E, Goh K, Berchel M et al. Molecular characterization and drug resistance patterns from patients in AIDS counselling centre in Portau- Prince, Haiti: a 1-year study. J Clin Microbiol 2003; 41: 694-702.
12. Groenen PWM, Bunschotten AEA, van Soolingen D, van Embden JD. Nature of DNA polymorphism in the direct repeat cluster of Mycobacterium tuberculosis. Application for strain differentiation by a novel method. Mol Microbiol 1993; 10: 1057-65.
13. Haas W H, Engelman G, Amthor B, Shyamba S, Mugala F, Felten M et al. Transmission dynamics of tuberculosis in a high-incidence country: prospective analysis by PCR-DNA fingerprinting. J Clin Microbiol 1999; 37: 3975-9.
14. Hanna B, Ebrahimzadeh A, Elliott B, Morgan M, Novak SM, Rusch-Gerdes S, et al. Multicenter evaluation of the Bactec MGIT 960 system for the recovery of mycobacteria. J Clin Microbiol 1999; 37: 748-52.
15. Heifets LB. Conventional methods for antimicrobial susceptibility testing of M. tuberculosis. In: Bastian I, Portaels F, editors. Multidrug-resistant tuberculosis. London, United Kingdom. Kluwer Academic Publishers, 2001, p. 135-6.
16. Instituto Nacional de Enfermedades Respiratorias Emilio Coni. Notificación de casos de tuberculosis en Argentina. Situación nacional y por provincias. 2003. PRO.TB.44/03. Santa Fe.
17. Kremer K, Glyn J, Lillebaek T, Nieman S, Kurepina N, Kreiswirth B et al. Definition of the Beijing/W lineage of Mycobacterium tuberculosis on the basis of genetic markers. J Clin Microbiol 2004; 39: 4040-9.
18. Kremer K, van Soolingen D, Frothingham R, Haas W H, Hermans P W, Martin C et al. Comparison of methods based on different epidemiological markers for typing Mycobacterium tuberculosis complex strains: interlaboratory study of discriminatory power and reproducibility. J Clin Microbiol 1999; 37: 2607-18.
19. Lockman S, Sheppard JD, Braden CR, Mwasekaga MJ, Woodley CL, Kenyon TA et al. Molecular and conventional epidemiology of Mycobacterium tuberculosis in Botswana: a population prospective study of 301 pulmonary tuberculosis patients. J Clin Microbiol 2001; 39: 1042-7.
20. Morcillo N, Alito A, Romano MI, Cataldi A, Dolmann, A, Reniero A, et al. Multidrug-resistant tuberculosis outbreak in Buenos Aires. DNA fingerprinting analysis of isolates. Medicina (Buenos Aires) 1996; 56: 45-7.
21. Morcillo N, Zumárraga M, Alito A, Dolmann A, Schouls L, Cataldi A, et al. A low cost, homemade, reverse-line blot hybridisation assay for rapid detection of rifampin resistance in Mycobacterium tuberculosis. Int J Tuberc Lung Dis 2002; 6: 959-65.
22. Pfyffer GE, Strassle A, Rose N, Wirth R, Brandli O, Shang H. Transmission of tuberculosis in the metropolitan area of Zurich: a three years survey based on DNA fingerprinting. Eur Respir J 1998; 11: 804-8.
23. Ritacco V, Di Lonardo M, Reniero A, Barrera L, Ambroggi M, Kantor IN de, et al. Nosocomial spread of human immunodeficiency virus-related multidrug-resistant tuberculosis in Buenos Aires. J Infect Dis 1997; 176: 637-42.
24. Small P, Hopewell PC, Singh SP, Paz A, Parsonnet J, Ruston DC et al. The epidemiology of tuberculosis in San Francisco: a population-based study using conventional and molecular methods. N Engl J Med 1994; 39: 1042-7.
25. Small P. Molecular strain typing of Mycobacterium tuberculosis to confirm cross-contamination in the mycobacteriology laboratory and modification of procedures to minimize occurrence of false-positive cultures. J Clin Microbiol 1993; 31: 1677-82.
26. Van der Spuy GD, Warren GD, Richardson M, Beyers N, Behr MA, van Helden PD. Use of genetic distance as a measure of ongoing transmission of M. tuberculosis. J Clin Microbiol 2003; 41: 5640-4.
27. Van Embden, JDA, Cave MD, Crawford JT, Dale JW, Eisenach KD, Gicquel B, et al. Strain identification of M. tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol 1993; 31: 406-9.
28. Van Soolingen D. Molecular epidemiology of tuberculosis and other mycobacterial infections. J Intern Med 2001; 249: 1-26.
29. Vynnycky E, Nagelkerke N, Borgodoff MW, van Soolingen D, van Embden JD, Fine PE. The effect of age and study duration on the relationship between "clustering" of DNA fingerprint patterns and the proportion of tuberculosis disease attributable to recent transmission. Epidemiol Infect 2001; 407: 43-62.
30. Warren RM, van der Spuy GD, Richardson M, Beyers N, Booysen B, Behr MA, et al. Evolution of IS6110-based restriction fragment length polymorphism patterns during the transmission of Mycobacterium tuberculosis. J Clin Microbiol 2002; 40: 1277-82.
31. World Health Organization. Laboratory Services in Tuberculosis Control. 1998. WHO/TB 98. 258 Geneva. [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ]
Recibido: 16/04/07
Aceptado: 31/07/07














