Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista argentina de microbiología
versión impresa ISSN 0325-7541versión On-line ISSN 1851-7617
Rev. argent. microbiol. v.40 n.1 Ciudad Autónoma de Buenos Aires ene./mar. 2008
Extracellular proteases from the Antarctic marine Pseudoalteromonas sp. P96-47 strain
S.C. Vázquez1, 3*, E. Hernández1, W.P. Mac Cormack2
1Cátedra de Microbiología Industrial y Biotecnología, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires. Junín 956 6º Piso (C1113AAD) Ciudad Autónoma de Buenos Aires;
2Instituto Antártico Argentino. Cerrito 1248 (C1010AAZ) Ciudad Autónoma de Buenos Aires;
3Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina.
*Correspondence. E-mail: svazquez@ffyb.uba.ar
ABSTRACT
The extracellular protease-production capacity of 33 bacterial isolates taken from marine biotopes in King George Island, Antarctica, was evaluated in liquid cultures. The P96-47 isolate was selected due to its high production capacity and was identified as Pseudoalteromonas sp. The optimal growth temperature was 20 °C and the optimal for protease production was 15 °C. Proteases were purified from culture supernatants, developing a multiple-band profile in zymograms. They were classified as neutral metalloproteases and worked optimally at 45 °C with an Eact of 47 kJ/ mol. Their stability was higher at neutral pH, retaining more than 80% of activity at pH 6-10 after 3 h incubation at 4 °C. After 90 min incubation at 40 and 50 °C, the percentages of residual activities were 78% and 44%. These results contribute to the basic knowledge of Antarctic marine proteases and also help evaluate the probable industrial applications of P96-47 proteases.
Key words: Pseudoalteromonas sp.; Antarctic bacteria; Proteases; Sychrotolerant bacteria; Sychrophiles
RESUMEN
Proteasas extracelulares de la cepa marina antártica Pseudoalteromonas sp. P96-47. La capacidad productora de proteasas extracelulares de 33 aislamientos bacterianos tomados de biotopos marinos en la Isla Rey Jorge, Antártida, fue evaluada en cultivo líquido. El aislamiento P96-47 fue seleccionado debido a su alta capacidad productora y fue identificado como Pseudoalteromonas sp. La temperatura óptima de crecimiento fue de 20 °C y la de producción de 15 °C. Las proteasas fueron purificadas a partir del sobrenadante de cultivo, y en los zimogramas desarrollaron un perfil de múltiples bandas. Estas proteasas fueron clasificadas como metaloproteasas neutras y se observó que trabajan óptimamente a 45 °C, con una Eact de 47 kJ/ mol. Su estabilidad fue superior a pH neutro y retuvieron más del 80% de su actividad a pH 6-10 después de 3 h de incubación a 4 °C. Luego de 90 min de incubación a 40 y 50 °C, las actividades residuales fueron 78% y 44%, respectivamente. Los resultados que se presentan en este trabajo contribuyen al conocimiento básico de las proteasas marinas antárticas y también a evaluar las probables aplicaciones industriales de las proteasas de P96-47.
Palabras clave: Pseudoalteromonas sp.; Bacterias antárticas; Proteasas; Bacterias psicrotolerantes; Psicrófilos
INTRODUCTION
Extreme environments are very particular sites. The microorganisms that are able to live under the harsh physicochemical conditions of those places have their biochemistry adapted to work optimally in the biotopes where they thrive. The Antarctic continent is one of the coldest areas on earth and in several of its environments (such as seawater, marine sediments and sea ice) the temperature is approximately constant throughout the whole year. In these places, bacterial microbiota is adapted to live in the cold and to resist freezing and thawing cycles. The enzymes produced by these microorganisms work more efficiently at low temperatures than the corresponding mesophilic and thermophilic ones (6, 9, 12).
In marine environments, several bacterial groups play a key role in the degradation of organic matter whereas others are responsible for a significant fraction of the primary production contributing to the cycling of C, N and P (3).The enzymes produced by these organisms take part in these processes and, therefore, their isolation and study are of great importance not only for the comprehension of their biochemical characteristics and their activity in situ but also for the probable relevance in many different industrial applications. Cold-active enzymes can be used in food processing, the leather industry, laundry powders for cold-water washing, waste treatment and as cleaning (2, 22).
The γ-Proteobacteria group comprises several genera which include species which are reported as dominant in marine environments (4). Among these genera, the Pseudoalteromonas species are worth noticing. The genus has several marine species which are able to produce a variety of bioactive compounds, including extra cellular enzymes. This ability enables them to compete for nutrients, colonize surfaces, live in association with other organisms and assimilate complex organic compounds (11). Extracellular proteases represent one of the most important groups of hydrolytic enzymes and the Pseudoalteromonas species are known to produce exoproteases, which mainly act in algae and organic matter degradation (13, 15, 16, 20).
Aiming at improving the basic knowledge on this matter and keeping in mind the industrial interest in coldadapted proteases, this work was conducted with the purpose of isolating and generally describing proteaseproducing marine bacteria from Antarctica, focusing on the characterization of the proteases produced by one Pseudoalteromonas sp. strain.
MATERIALS AND METHODS
Isolation of microorganisms
Isolates were obtained from samples of marine water, sediment or dead marine animals as previously described (23, 24).
P96-47 strain was selected among 33 marine proteaseproducing isolates as a producer of high levels of cold-adapted proteases in submerged culture. This strain was identified at genus level by using molecular techniques, as elsewhere described (19).
Bacterial cultures
The strains were grown in marine nutrient broth CM1 (Oxoid, Ltd, Basingstoke, Hampshire, England) supplemented with 0.3 g/l CaCl2.2H2O, pH 8.0 and prepared with 100% v/v artificial seawater (17). From each isolate, duplicate cultures of 60 ml in 300-ml flasks were inoculated with 1% (v/v) of a subculture and incubated at 20 °C and 240 rpm on a rotary shaker until they reached the stationary phase of growth (24). The effect of temperature on growth and protease secretion was evaluated on cultures incubated at 15, 20 and 28 °C. The maximal specific growth rate (µmax) at each temperature was established by linear regression analysis in order to determine the slope of the linear part of the semilogarithmic plot of dry weight against time. Protease secretion at each time was estimated by measuring the proteolytic activity in the culture supernatants of the samples, as described below.
Protease detection
Proteolytic activity was measured by digestion previously of azocasein (Sigma, St.Louis, USA) according to the method previously described (24). One EU was defined as the amount of enzyme that produces an increase of 0.100 in A340 under the assay conditions. Two commercial proteases were used as standard: Metalloprotease Type IX P6 141 (Sigma) and Subtilisin Type VIII P5380 (Sigma, St. Louis, USA). Protease profiles were developed in zymograms after electrophoresis (SDS-PAGE) of cell-free culture supernatants, using gelatin as a copolymerized substrate (10). Proteolytic activity was evident as gelatin-depleted bands.
Biochemical purification
For their characterization, a solution containing the P96-47 proteases was obtained from culture supernatant by concentrating the fluid by tangential ultra filtration and subsequent passage through a XK 26/20 (Amersham Pharmacia Biotech, Uppsala, Sweden) column containing S-Sepharose Fast Flow resin as previously described (25). Other purification methods were tried, such as hydrophobic interaction chromatography (HIC) with Phenyl Sepharose and Octyl Sepharose resins, immobilized metal ion affinity chromatography (IMAC) with Ni2+, Cu2+ and Zn2+ ions, anion exchange chromatography (AEC) with Q (pH 10.0) and DEAE (pH 8.0 and 9.0) resins and cation exchange chromatography (CEC) with Sulfopropyl resin (pH 5.0). These methods were not able to separate active bands and, in addition, gave a low or null recovery of the proteolytic activity. Total protein content was measured by the bicinchonic acid method (21) using the commercial kit BCA (Pierce, Rockford, Illinois, USA). Purity was checked before and after purification by non-reducing SDS-PAGE stained with Coomassie Blue R250 0.1% in a solution of 30:10:60 methanol, acetic acid, water.
Effect of temperature and pH on activity and stability
The effect of pH on protease activity and stability was determined by using the standard protease assay as previously described (24). For determination of the apparent optimal temperature (OT), the proteolytic activity was measured at various temperatures ranging from 0 °C to 60 °C and pH 8.0. Apparent activation energies (Eact) were calculated from the linear portion of Arrhenius plots as described by Pirt (18). For the evaluation of the effect of temperature on enzyme stability, the proteases were incubated at pH 8.0 for 1 h (enzyme assay buffer) at temperatures ranging from 10 to 60 °C. Following, the enzyme solutions were cooled and the residual activity was measured at 20 °C using the standard protease assay. In addition, the proteases were incubated at 40 °C and 50 °C for various periods. Afterwards, the mixtures were rapidly cooled and the residual activities were determined by using the standard protease assay. The half time of thermal inactivation (ti) was calculated as the incubation time at which the protease retains 50% of its maximal activity at a given temperature (14).
Effect of inhibitors and metal ions
The effect of protease inhibitors (10 mM each): PMSF, 2,2- bipyridil, 1,10-phenantroline, EDTA and cystein, as well as of metal ions (1 mM each): Zn2+, Hg2+, Cu2+ and Ni2+ was investigated. The proteases were incubated with each of the reagents at 20 °C and pH 8 for 1 h. The residual activities were determined by using the standard protease assay.
RESULTS
Characterization of strains
Of the 33 heterotrophic, aerobic, psychrotolerant marine isolates, the majority of them were non-spore forming gram-negative rods (Table 1). All of them were able to produce extracellular proteases detected as clear zones of casein hydrolysis around colonies in skim-milk agar plates. The P96-47 isolate was the best protease producer and was selected for the characterization of its proteases. It was identified at genus level by comparison of the partial sequence (5'-end, 493 bp) of the 16S-rDNA gene with sequences deposited in the databases. P96- 47 was identified as Pseudoalteromonas sp., with 98.3% identity with Pseudoalteromonas sp. AS-41, AJ391202 and 96.9% identity with Pseudoalteromonas aliena KMM 3562, AY387858 (type strain) in the RDP database (1).
Table 1. Growth and proteolytic activity levels reached after 72 h of culture of the Antarctic marine bacteria isolated from different samples and optimal temperature for activity (TO) and activation energies (Eact) of the proteases produced. Values are expressed as the average of duplicates ± SD.

Growth, protease secretion and temperature dependence of proteases
When all the isolates were grown at 20 °C in liquid culture, the maximal growth was observed after 24-48 h of incubation. Also, protease secretion started at the be ginning of the stationary phase of growth, reaching its maximum in the late stationary phase (48-72 h), for almost all the isolates. In any culture, the increase in proteolytic activity correlated with a decrease in biomass levels, suggesting that the activities appearing in culture supernatants did not come from inside the cells as a result of bacterial lysis. The levels of biomass and proteolytic activity at the end of cultures are shown in Table 1. Three isolates failed to secrete detectable amounts of protease, even when they produced casein hydrolysis in skim-milk agar plates.
The dependence of proteolytic activity on temperature for hydrolysis of azocasein was determined for the cellfree culture supernatants after centrifugation of cultures. The optimal temperature for activity was determined between 40 and 50 °C for all proteases. The Eact values for proteolytic activity were variable and, for some crude protease extracts, about 10-20 kJ/mol lower than those observed for the mesophilic commercial protease Subtilisin (60 kJ/ mol). The pattern of proteolytic activity developed by culture supernatants on gelatin-SDS-PAGE mainly showed multiple-band profiles, with only two isolates from a dead elephant seal skin, which revealed only one band.
On the basis of its behavior towards temperature of the proteases present in crude extract and the considerable high levels of proteolytic activity detected at the end of culture, the P96-47 isolate was selected for further characterization of the proteases produced. P96-47 was able to grow from 15 to 28 °C but not at 34 °C, proving to be psychrotolerant. The optimal growth temperature was 20 °C (growth rate µmax 1.4 /h, biomass 2.6 g/l), but the highest proteolytic activity in culture supernatant (63.2 EU/ml) and the maximum protease production yield (33.3 EU/g) were reached at 15 °C.
Preparation of P96-47 proteases for further characterization
After production in liquid culture at 20 °C for 72 h, reaching a proteolytic activity of 67.1 EU/ml, the proteases present in culture supernatant were concentrated by tangential ultra filtration with a recovery of 100% of proteolytic activity and a purification factor of 1.5. The concentrate was further purified to separate the contaminant proteins (components of culture media and other bacteriaderived proteins without proteolytic activity) by cation exchange chromatography and gel filtration with a recovery in protease activity of 27% (2.4-fold final purification). The specific activity of proteases in the obtained solution was of 4330 EU/mg protein.
After trying several methods (mentioned in the Materials and Methods section), no one was able to separate the different active bands observed in zymograms. Ssepharose chromatography was finally used because it gave the best results (higher recovery and concentration of proteolytic activity). The major peak of proteins that appeared in the elution profiles was coincident with the peak with proteolytic activity. Nevertheless, as mentioned above, SDS-PAGE and zymogram of the single peak with proteolytic activity showed that the methods used were effective in removing most of the contaminating proteins but not in separating the five active bands present in the zymogram of crude extracts (Figure 1). These active bands had molecular masses of 52, 43.5, 41, 40 and 30.5 kDa. Even when the bands developed in the zymograms were clearly visible, no bands with molecular masses corresponding to those of the proteases could be observed in SDS-PAGE of the culture supernatant, which indicated that proteases have high activity but are present at low mass concentration. This fact made it necessary to concentrate the supernatant by ultrafiltration and to subsequently apply a purification method that concentrates the sample instead of other methods such as gel filtration, which dilute the purified proteins. As a result, a solution containing a mixture of proteins with proteolytic activity (that could not be separated) but without the contaminant proteins coming from the culture media was obtained and further characterized as a whole.

Figure 1. (a) Zymogram (SDS-PAGE with copolymerized gelatin) of P96-47 proteases present in culture supernatant. Gel was stained with Amidoblack 1% w/v in a solution of 30:10:60 methanol, acetic acid, water, (b) Non-reducing SDS-PAGE of concentrated culture supernatant (1) and of the pooled fractions with proteolytic activity after cation exchange chromatography (2). Gels were stained with Coomassie Blue R250 0.1% in a solution of 30:10
Effect of temperature and pH on activity of P96-47 proteases
For P96-47, the optimal proteolytic activity was registered at neutral and slightly alkaline pH. The pH-dependence profile showed maximal activity between pH 7.0 and pH 9.0, with relative activity above 50% between pH 6.0 and 10.0, and more than 80% between pH 7.0 and 9.0 (Figure 2a).

The thermal-dependence profile of P96-47 proteases appears to have more than one peak, probably corresponding to the response of more than one protease with different temperature dependence for their activity. The apparent optimum temperature for activity towards azocasein of the purified proteases was observed at 45 °C, a lower value than those registered for the commercial proteases used as reference. One important characteristic is that the proteases displayed above 70% of relative activity on the broad range of temperatures comprised between 35 °C and 60 °C (Figure 2b). The Arrhenius law was followed between 10 and 45 °C and the apparent Eact was 47 kJ/mol. This value is lower than those obtained from the commercial mesophilic Metalloprotease (58 kJ/mol) and the subtilisin (60 kJ/mol), tested under the same conditions.
Effect of pH and temperature on stability of P96-47 proteases
The stability of P96-47 proteases was higher at neutral pH, retaining more than 80% of the maximal activity at pH ranging from 6.0 to 10.0 after incubation of 3 h at 4 °C. At pH 5.0 and 11.0, 60-70% of relative activity was still conserved, being the P96-47 proteases more active at pH 4.0 than the commercial metalloprotease and subtilisin, tested under the same conditions. Nevertheless, at pH 12.0, only 10% of relative activity could be detected, as well as for the neutral metalloprotease, while the alkaline subtilisin was 75% active (Figure 3a).

The stability after 1 h exposure to different temperatures was similar to that observed for the metalloprotease, but lower than that displayed by the subtilisin. The maximal activity was only retained at temperatures below 30 °C, only 50% was retained at 45 °C and only 10% at 60 °C, with a rapid thermal inactivation (Figure 3b). After 90 min of incubation at 40 and 50 °C, the residual activities were 78% and 44% for P96-47 proteases, while subtilisin retained 90% and 65% of its initial activity. On the other hand, the metalloprotease retained 82% and 24%, being more sensitive at 50 °C than the psychrotolerant proteases (Figure 4a and 4b). At this temperature, the ti of P96-47 proteases was of 65 min, while the metalloprotease had a lower ti of 22 min and the subtilisin a higher value, longer than 90 min (the final time of the assay).

Effect of inhibitors and metal ions on activity of P96-47 proteases
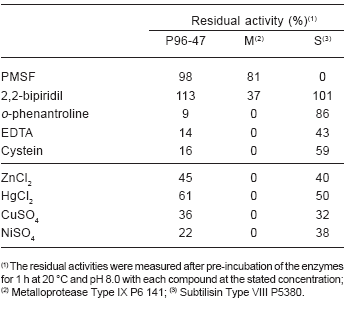
The solution of purified proteases was 91% inhibited by 10mM 1, 10-phenantroline and 86% inhibited by EDTA (metalloprotease inhibitors), which indicated the presence of metalloproteases. PMSF and 2, 2-bipyridil did not affect the enzyme and cystein caused 84% of inhibition of the activity, probably due to the presence of disulphide bonds essential for keeping an active enzyme conformation. The presence of 1 mM Zn2+, Hg2+, Cu2+ and Ni2+ affected the activity of the enzyme solution by 39-78%, slightly higher than the inhibition of the commercial subtilisin tested under the same conditions, while the metalloprotease was completely inhibited (Table 2).
Table 2. Effect of inhibitors and metal ions on the activity of proteases from the Antarctic strain Pseudoalteromonas sp. P96-47. :60 methanol, acetic acid, water.
DISCUSSION
The description and characterization of the enzymes produced by extremophilic organisms is relevant not only for their participation in the natural cycles in the environment but also for their possible application in industry. The exploration of biodiversity can lead to the discovery of not-yet-described enzymes or to the elucidation of the role enzymes play in their natural biotopes. Bacteria inhabiting marine environments often produce exoenzymes that help them take advantage of macromolecules as nutrients. Among the proteolytic marine bacteria isolated from water, sediment and animal samples, gram-negative rods were predominant. The same was observed for non-marine environments within the same geographic area (24). Unexpectedly, the proteases had optimal activity at temperatures up to 50 °C higher than the temperatures where the producing organisms live. Also the Eact found were in general slightly lower than the values registered for mesophilic proteases. Even when the natural environment of the producing bacteria never reaches temperatures higher than 10 °C, the proteases produced by them are not fully adapted to work in the cold, having temperature dependence for activity similar to that found for proteases produced by mesophilic bacteria. Nevertheless, even when the temperature dependence curves showed that in situ, the levels of proteolytic activity are almost negligible at the temperature of the environment where the producing strains live, nothing can be said about the performance of the enzymes in their natural environment, which could be better than that displayed by them when tested in vitro.
The species comprised in the genus Pseudoalteromonas, which exclusively thrive in marine waters all around the world, have diverse adaptive and survival strategies. They are also frequently found associated to eukaryotic organisms or to particles of organic matter and the broad spectrum of bioactive metabolites that they can produce target a great number of organisms (11). Bacterial psychrophilic and psychrotolerant strains of marine origin, belonging to the genus Pseudoalteromonas, have been found to produce a variety of enzymatic activities. Strains isolated from deep-sea sediment produce coldactive cellulose (27) and protease (8). A cold β-galactosidase from an Antarctic Pseudoalteromonas haloplanktis was extensively characterized and produced as a recombinant protein in Escherichia coli displaying no alterations in its properties (12). A protease with tolerance to high salt concentrations is produced in high amounts by a Pseudoalteromonas sp. strain isolated from Spanish hypersaline environments (20). Another strain associated with the brown algae Fucus evanescens has proved to produce a variety of metabolic activities (bacteriolytic and hemolytic activities, gelatinases and caseinases, lipases, DNAses, fucoidanhydrolases, laminarases, alginases, pustulanases, β-glucosidases, β-galactosidases and β-xylosidases) (13). Furthermore, other strains were found to live in association with higher organisms and to produce inhibitory extracellular agents as well as compounds which promote the survival of other
Enzymes produced by Pseudoalteromonas and other microorganisms during their descent through the water column, contribute to the progressive degradation of the organic matter in the sea. They also take part in the mineralization processes, which occur close to the bottom, as in the degradation of detritus in the benthic layer. Proteases from Pseudoalteromonas species participate in the lysis of marine algae, as it is described for a strain isolated from the brown algae Fucus evanescens which plays a role in the initial stages of algal degradation (13).
The genus Pseudoalteromonas is apparently composed of good producers of a variety of metabolites. It is reported that the strain Pseudoalteromonas sp. CP76 was selected as the best protease producer among several moderate halophiles (20). In this work, P96-47 was selected for being the best producer among all the proteolytic isolates. When growth and protease production were tested at 15, 20 and 28 °C, maximal proteolytic activity occurred at 15 °C, while the highest growth rate and maximal biomass were recorded in cultures incubated at 20 °C, which indicates that P96-47 is psychrotolerant and that its best physiological condition is reached at temperatures well below the optimal temperature for activity of the produced proteases (45 °C).
As mentioned above, though P96-47 displayed high levels of protease production in cultures incubated at low temperature, the optimum temperature for activity of its proteases is slightly lower than that of mesophilic metalloproteases, retaining up to 80% of maximal activity at 50-60 °C. This fact tells about the adaptation of P96- 47 isolate to live in the cold relying more on the increase of the amount of high-specific-activity proteases produced, than on the ability of its enzymes to efficiently work at low temperatures. This probable strategy was also observed for the proteases produced by three Stenotrophomonas maltophilia strains isolated from sub Antarctic soils (26), in contrast with other proteolytic isolates from the same area which display optimal activity at lower temperatures than those found for the corresponding mesophiles (23, 24). Regarding this strategy, Feller and Gerday (5) mentioned that in order to compensate for the low kinetic energy at low temperatures and, thus, support nutrient degradation and uptake, psychrophilic and psychrotolerant microorganisms can either synthesize cold-efficient enzymes or produce high levels of enzymes. In addition, the thermal dependence curve obtained for P96-47 proteases seemed to be shaped for two superimposed curves (the activity at 60 °C was confirmed by repeating the measure three independent times), originated from the activity of different enzymes in the solution obtained after purification, which also revealed a multiple band profile in zymograms that may correspond to more than one protease and/or to isozymes. The producing strain was isolated from surface sea water. The marine environment is an oligotrophic habitat where the biosynthesis of more than one enzyme of a given catalytic type can benefit the bacteria with the advantage of the ability of using a broad spectrum of substrates with different efficiencies. From an industrial point of view, the relevance of this aspect relies on the fact that the production of proteases with the P96-47 strain yields a preparation that retains up to 70% of its maximal activity in a broad range of temperatures (35-60 °C).
Usually, bacterial neutral metalloproteases are active in a narrow pH range (5 to 8) and have relatively low thermotolerance. Nevertheless, the P96-47 proteases cover a broad pH range of activity with more than 50% of relative activity between pH 5 and 11, which extends the spectrum of possible industrial applications. The thermal lability can be a desired property in some industrial biotechnological processes, as they generate less bitterness in hydrolyzed food proteins than animal proteases do, and their reactivity can be controlled to produce food hydrolysates with a low degree of hydrolysis. They can also be selectively inactivated after working in a complex mixture of enzymes or after cooking in the case of food manufacturing processes. The thermal instability displayed by the Antarctic proteases herein described is not surprising, since cold-adapted enzymes have more flexible tertiary structures that confer higher catalytic efficiency at the biotope temperatures where the producing organisms thrive, which allows higher reaction velocity and substrate turnover, but can also lead to the risk of thermal stability loss (7).
To conclude, the information herein presented contributes to the general knowledge on marine cold-active proteases. Gathering the information about the characteristics of the bioactive compounds produced in marine environments for the different organisms will allow the elucidation of the dynamics of the organic cycles and food webs. On the other hand, and regarding the industrial use of enzymes, having the basic knowledge on the characteristics of the enzymes found in nature and the molecular adaptations to the conditions of the biotopes where they naturally work is required in order to apply molecular techniques to modify already commercialized enzymes or newly isolated ones. On the basis of this knowledge, modern biotechnology can alter thermal stability, pH and OT, substrate specificity or even yields of production in order to improve the enzyme performance to cover desired applications.
Acknowledgements: We gratefully acknowledge the contribution of Dr. Marta Mollerach and Dr. Gabriel Gutkind and all the people at the Culture Collection CCM029, Cátedra de Microbiología, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires for their assistance in the molecular identification of the strain and of German Warckmeister for the revision of the English manuscript. This work was carried out under an agreement between Instituto Antártico Argentino and Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires and supported in part by the UBACyT TB-31 and IAA 41 grants.
1. Cole JR, Chai B, Farris RJ, Wang Q, Kulam SA, McGarrell DM, et al. The Ribosomal Database Project (RDP-II): sequences and tools for high-throughput rRNA analysis. Nucleic Acids Res 2005; Jan 1:33 (Database Issue):D294- D296 doi: 10.1093/nar/gki038. [ Links ]
2. Demain AL. Stunning achievements of Industrial Microbiology. The News Magazine Am Soc Microbiol 1999; 65: 311-16. [ Links ]
3. Deming JW. Psychrophiles and polar regions. Curr Opin Microbiol 2002; 5: 301-9. [ Links ]
4. Eilers H, Pernthaler J, Glöckner FO, Amann R. Culturability and in situ abundance of pelagic bacteria from the North Sea. Appl Environ Microbiol 2000; 66: 3044-51. [ Links ]
5. Feller G, Gerday C. Psychrophilic enzymes: molecular basis of cold adaptation. CMLS Cell Mol Life Sc 1997; 53: 830-41. [ Links ]
6. Feller G, Zekhnini Z, Lamotte-Brasseur J, Gerday Ch. Enzymes from cold adapted microorganisms. The class C b-lactamase from the Antarctic psychrophile Psychrobacter immobilis A5. Eur J Biochem 1997; 244: 186-91. [ Links ]
7. Feller G. Molecular adaptations to cold in psychrophilic enzymes. CMLS Cell Mol Life Sc 2003; 60: 648-62. [ Links ]
8. He H, Chen X, Li J, Zhang Y, Gao P. Taste improvement of refrigerated meat treated with cold-adapted protease. Food Chem 2004; 84: 307-11. [ Links ]
9. Helmke E, Weyland H. Effect of temperature on extracellular enzymes occurring in permanently cold marine environments. In: Rheinheimer G, Gocke K, Hoppe H, Lochte K, Meyer-Reil LA, editors. Distribution and activity of microorganisms in the sea. Proceedings of the 4th European Marine Microbiology Symposium. Kiel, Germany, Universität Kiel, 1991, p. 198-204. [ Links ]
10. Heussen C, Dowdle EB. Electrophoretic analysis of plasminogen activators in polyacrilamide gels containing sodium dodecyl sulfate and copolymerised substrates. Anal Biochem 1980; 102: 196-202. [ Links ]
11. Holmström C, Kjelleberg S. Marine Pseudoalteromonas species are associated with higher organisms and produce biologically active extracellular agents. FEMS Microbiol Ecol 1999; 30: 285-93. [ Links ]
12. Hoyoux A, Jennes I, Dubois P, Genicot S, Dubail F, Francois JM, et al. Cold-adapted b-galactosidase from the Antarctic psychrophile Pseudoal-teromonas haloplanktis. Appl Environ Microbiol 2001; 67: 1529-35. [ Links ]
13. Ivanova EP, Bakunina IY, Sawabe T, Hayashi K, Alexeeva YV, Zhukova NV, et al. Two species of culturable bacteria associated with degradation of brown algae Fucus evanescens. Microb Ecol 2002; 43: 242-9. [ Links ]
14. Kärst U, Woehl M, Czempinski K, Schmid R D. Characterization of extracellular hydrolases from marine psychrophilic bacteria. In: Alberghina L, Frontali L, Sensi P, editors. Progress in biotechnology: proceedings of the 6th European Congress on Biotechnology. Florence, 1993. Amsterdam, Elsevier Science, 1994, p. 263-6. [ Links ]
15. Lee S, Kato J, Takiguchi N, Kuroda A, Ikeda T, Mitsutani A, et al. Involvement of an extracellular protease in algicidal activity of the marine bacterium Pseudoalte-romonas sp. Strain A28. Appl Environ Microbiol 2000; 66: 4334-9. [ Links ]
16. Lovejoy C, Bowman JP, Hallegraeff GM. Algicidal effects of a novel marine Pseudoalteromonas isolate (class Proteobacteria, gamma subdivision) on harmful algal bloom species of the genera Chattonella, Gymnodinium and Heterosigma. Appl Environ Microbiol 1998; 64: 2806-13. [ Links ]
17. Lyman J, Fleming RH. Composition of seawater. J Mar Res 1940; 3: 134-6. [ Links ]
18. Pirt SJ. Effects of temperature. In: Pirt SJ, editor. Principles of microbial and cell cultivation. London, Blackwell, 1985, p. 137-42. [ Links ]
19. Ruberto L, Vázquez SC, Mac Cormack WP. Effectiveness of the natural bacterial flora, biostimulation and bioaugmentation on the bioremediation of hydrocarbon contaminated Antarctic soil. Int Biodeterior Biodegrad 2003; 52: 115-25. [ Links ]
20. Sánchez-Porro C, Mellado E, Bertoldo C, Antranikian G, Ventosa A. Screening and characterization of the protease CP1 produced by the moderately halophilic bacterium Pseudoalteromonas sp. strain CP76. Extremophiles 2003; 7: 221-8. [ Links ]
21. Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD et al. Measurement of protein using bicinchonic acid. Anal Biochem 1985; 150: 76- 85. [ Links ]
22. Van den Burg B. Extremophiles as a source of novel enzymes. Curr Opin Microbiol 2003; 6: 213-8. [ Links ]
23. Vázquez SC, Ríos Merino L N, Mac Cormack WP, Fraile ER. Protease-producing psychrotropic bacteria isolated from Antarctica. Polar Biol 1995; 15: 131-5. [ Links ]
24. Vázquez SC, Mac Cormack WP. Effect of isolation temperature on the characteristics of extracellular proteases produced by Antarctic bacteria. Polar Res 2002; 21: 63-71. [ Links ]
25. Vázquez SC, Coria SH, Mac Cormack WP. Extracellular proteases from eight psychrotolerant Antarctic strains. Microbiol Res 2004; 159: 157-66. [ Links ]
26. Vázquez SC, Ruberto L, Mac Cormack WP. Properties of extracellular proteases from three psychrotolerant Stenotrophomonas maltophilia isolated from Antarctic soil. Polar Biol 2005; 28: 319-25. [ Links ]
27. Zeng R, Xiong P, Wen J. Characterization and gene cloning of a cold-active cellulase from a deep-sea psychrotrophic bacterium Pseudoalteromonas sp. DY3. Extremophiles 2006; 10: 79-82. [ Links ]
Recibido: 19/02/07
Aceptado: 27/11/07














