Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista argentina de microbiología
versión impresa ISSN 0325-7541versión On-line ISSN 1851-7617
Rev. argent. microbiol. v.40 n.3 Ciudad Autónoma de Buenos Aires jul./sep. 2008
Retrospective study of bovine neonatal mortality: cases reported from INTA Balcarce, Argentina
E. L. Morrell1, D. P. Moore1, 2, A. C. Odeón1, M. A. Poso1, E. Odriozola1, G. Cantón1, F. Paolicchi1, R. Malena1, M. R. Leunda1, C. Morsella1, C. M. Campero1*
1 Animal Health Group, Instituto Nacional de Tecnología Agropecuaria (INTA), Balcarce;
2 Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina;
*Correspondence. E-mail: ccampero@balcarce.inta.gov.ar
ABSTRACT
A retrospective study was performed on 169 beef and dairy calves aged from 1 to 7 days old submitted to the Diagnostic Laboratories at INTA Balcarce, Argentina. Bacterial culture was performed for aerobic and microaerophilic organisms. Samples from spleen and lymph nodes, and peripheral blood mononuclear cells were also cultured for viral isolation on cell culture. Bovine rotavirus was detected by direct-ELISA. Multiple tissue samples were fixed in 10% formalin, routinely processed and stained with hematoxylin and eosin for microscopic examination. Etiological diagnosis was made in 70 of the 169 calves. Infectious agents were identified in 49 cases, the most common being Escherichia coli. When the histopathological examination was performed in cases with undetermined diagnosis, it was noted that 44 specimens had histological lesions, which suggested the presence of an infectious agent. In order to characterize the causes of bovine neonatal mortality, the protocols and methodology should be improved in further works.
Key words: Bovine; Neonatal mortality; Diagnosis; Beef; Dairy
RESUMEN
Estudio retrospectivo de mortalidad neonatal bovina. Casos hallados en INTA-Balcarce, Argentina. Se realizó un estudio restrospectivo en 169 terneros muertos 1 a 7 días después del nacimiento pertenecientes a rodeos para carne y leche, remitidos a los Laboratorios de Diagnóstico del INTA Balcarce, Argentina. Para detectar organismos aeróbicos y microaerófilos se realizó el cultivo bacteriano. Para el aislamiento viral sobre cultivo celular, se recolectaron muestras de bazo, ganglios linfáticos y sangre periférica. El rotavirus bovino fue identificado por ELISA directo. Se efectuó el examen microscópico de diferentes tejidos, los cuales fueron fijados en formol al 10%, procesados y teñidos con hematoxilina y eosina. Se obtuvo un diagnóstico etiológico en 70 de los 169 terneros. Se identificaron agentes infecciosos en 49 casos, siendo el más común Escherichia coli. En los casos con diagnóstico indeterminado, el examen histopatológico realizado determinó que 44 especímenes poseían lesiones compatibles con la presencia de agentes infecciosos. Es necesario mejorar los protocolos y las metodologías de trabajo a los fines de caracterizar las causas de mortalidad neonatal en bovinos.
Palabras clave: Bovino; Mortalidad neonatal; Diagnóstico; Carne; Leche
INTRODUCTION
Bovine neonatal mortality has generated several studies worldwide (1, 4, 8, 27). Environment, management, and presence of pathogenic agents are critical factors involved in bovine neonatal mortality (8, 19, 26). Characterization of its causes is an important step in controlling and preventing productive and economic losses in the bovine industry (1, 4, 5).
In Argentina, beef cattle are usually raised in extensive grazing systems. In contrast, intensive farming methods are used for dairy herds (11, 15). Reproductive and productive losses resulting from infectious, metabolic, toxic and genetic diseases have a considerable economic impact on both beef and dairy herds (7). Although, several causes have been associated with bovine neonatal mortality worldwide (1, 4, 5, 8), regional data is scant. The aim of this retrospective study was to characterize the most relevant findings of bovine neonatal mortality in beef and dairy herds according to the INTA Balcarce records.
MATERIALS AND METHODS
Background and origin of the specimens
Calves and various specimens (tissue samples, sera, feces and intestinal content) were submitted to the Diagnostic Laboratories at INTA, Balcarce, from 1994 to 2005 for diagnostic purposes. The referred cases came from 61 commercial beef herds (Aberdeen Angus, Hereford breeds and their crossbreeds) and 15 dairy premises (Holstein and Jersey breeds) located in 37 counties from the pampas in Buenos Aires province. The period of neonatal mortality (hebdomadal period) was defined as calf loss occurring between 1 and 7 days of life (24). Morbidity and mortality rates were not recorded in the present work.
Necropsy procedures
Standard necropsy procedures were performed, and breed, sex and age were recorded (25). Data regarding the age of death, when available, was provided by the private practitioner. In addition, the postmortem findings such as thrombus in umbilical artery, aerated lungs, presence of colostrum and/or milk in digestive tract, and body fat metabolism were used to determine the cause of neonatal death as was mentioned by Wikse et al. (1994). One hundred and forty seven of the 169 (86.9%) calves had died spontaneously, the remainders were euthanized due to agonic stage and/or severe hypothermia. All animal experiments were conducted in accordance with the guidelines of the National Service of Animal Health.
Samples and laboratory procedures
Cultural isolation and characterization of bacteria were performed by standard methods. Briefly, samples from several tissues (lungs, liver, intestine, spleen, mesenteric lymph node gall bladder and synovial fluid) were cultured aerobically on 5% horse blood agar and MacConkey agar. Lung was also cultured microaerophilically both on 5% horse blood agar for Brucella spp. (3) and Skirrow agar and incubated for 7 days at 37 °C for Campylobacter spp. (13, 16). Selection of the samples for specific culture was based on previous herd antecedents and gross necropsy findings. No attempt was made to identify Clostridium perfringens, mycoplasmas and chlamydias.
Spleen and lymph node samples and peripheral blood mononuclear cells were also subjected to viral isolation. Briefly, a 10% tissue homogenate was prepared and inoculated onto cultures of Madin Darbin Bovine Kidney (MDBK) cells. After four blind passages, inoculated cultures were tested for bovine viral diarrhea virus (BVDV) and bovine herpes-virus (BHV) antigens by indirect fluorescent antibody tests (IFAT) with commercially available polyclonal antibodies (American Bio-Research, Sevierville, TN, USA). In addition, a direct-ELISA was performed in feces for bovine rotavirus identification (17). Diagnosis of bovine coronavirus was disregarded in this study due to its low incidence in Argentina and because this virus affects clinically mostly 2-3 week old calves (34).
Tissue samples were taken from brain (cortex, midbrain, medulla and cerebellum), adrenal glands, heart, kidney, liver, lung, lymph nodes, skeletal muscle, spleen and thymus for histological examination. Samples of all tissues with macroscopic pathological changes were also taken for histopathological analysis. Tissues were fixed in neutral-buffered 10% formalin, routinely processed and stained with hematoxylin and eosin. Kidney and liver smears were only taken from specimens having macroscopic lesions compatible with Leptospira spp. (i.e., the presence of jaundice and/or macroscopic lesions on liver and kidney) and processed by a direct fluorescent antibody test (DFAT) using a commercial conjugated antibody (rabbit antiserum NVLS, Ca, USA) as described (20). Samples were selected for laboratory analysis according to herd antecedents or based on observations during macroscopic examination.
Diagnosis criteria
The diagnostic findings were categorized in groups according to the necropsy, histopathological and microbiological findings as previously described by the authors (14). After that, all the specimens submitted were classified as determined (infectious and non-infectious conditions) and undetermined cases (with and without histological lesion).
Data Analysis
Data analysis regarding age of death were carried out by comparison of mean pairs using the Student's t test. Causes of death (determined or undetermined), death day, sex, and herd origin were assessed by the Chi square test. The number of cases for each etiological agent was analyzed by tests of proportions. Statistical analyses were performed using SAS Software v.6.0 (SAS Institute Inc.) (35). All statistical analyses were considered significant when p < 0.05.
RESULTS
All calves were Bos taurus, being beef genotype (Aberdeen Angus, Hereford and crossbreed) in 122 (72.2%) cases, and dairy genotype (Holstein and Jersey) in 38 (22.5%) cases. The genotype was not recorded in 9 (5.3%) cases.
Eighty five (50.3%) of calves were males whereas 67 (39.6%) were females. Sex was not recorded in 17 (10%) cases. There were significant statistical differences between the number of females and males according to their origin p < 0.05, being 62 males and 47 females from the beef herds, and 19 males and 18 females from the dairy herds. Data regarding sex and/or origin were not available in 23 cases.
Average age at time of death was 3.2 ± 2.3 days in beef calves and 4.9 ± 2.4 days in dairy calves p < 0.05. Calf's age was not recorded in 21 cases. Although arbitrarily grouped, there were significant differences in the number of calves dying between days 1 and 4 compared with those between days 5 and 7 ( p < 0.05). Most calves died between days 1 and 4 after birth (Figure 1). The number of beef calves dying between days 1 and 4 was higher than that recorded for dairy calves during the same period ( p < 0.05) (Figure 1). In contrast, little variation was recorded between beef and dairy calves dying between days 5 and 7.
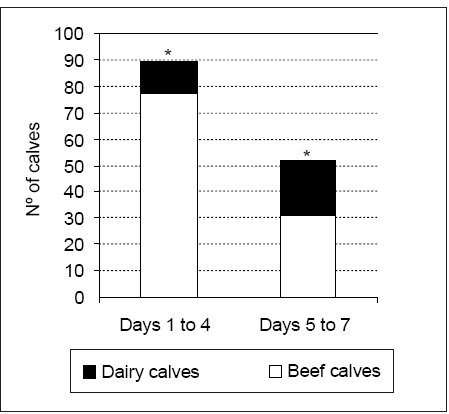
Figure 1. Number of deaths in beef and dairy calves arbitrary grouped in days 1 to 4 and 5 to 7, * p < 0.05.
Moderate to severe autolysis was present in spontaneously dying calves. Gross pathological findings including fibrinous pleuritis and/or pericarditis, peritonitis, pneumonia, enteritis, meningitis, icterus and congenital defects were seen at necropsy. In addition, starvation diagnosis (4 calves) was performed by the appearance of the epicardial and perirenal fat that changed from white and firm to brown-red and gelatinous and presence of grass and/ or soil in the abomasum instead of milk or colostrum. Hairballs were found in the abomasum of 2 beef calves with seven-day death. Severe signs of dehydration, diarrhea, dyspnea, anorexia, lethargy, stupor and coma were also seen.
Based on the anamnesic data submitted by the practitioner and changes observed during gross examination, samples were obtained for diagnosis. According to that criterion, bacterial cultures were requested in 97 (57.4%) cases, viral isolation or identification was solicited in 90 (53.3%) cases, and histological studies were performed on 121 (72.3%) cases.
Non-specific microscopic lesions possibly related to an infectious cause were observed in several tissues. Suppurative bronchopneumonia and/or enteritis were seen. Large numbers of neutrophils and macrophages were present in necrotic enteritis, fibrinous pericarditis, and multifocal necrotizing hepatitis. Epithelial necrosis with hemorrhagic lesions and cyst glands were also seen in intestine. Leukocytes filled the airspace, and bronchi or bronchioles were present in cases of pneumonia. Additionally, suppurative meningitis was noted in cases with septicemic form. Presence of leptospires on kidney smears was associated with non-suppurative multifocal nephritis. Frequency of histopathological lesions found in several organs is shown in Figure 2.
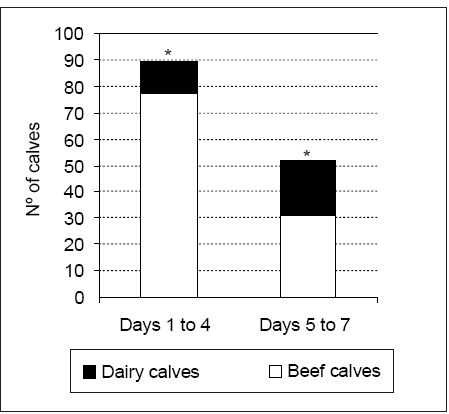
Figure 2. Frequency of histopathological lesions according to the affected organs.
Table 1 summarizes the diagnostic results. A specific cause of neonatal mortality was established in 70/169 (41.4%) cases; however, it was statistically lower than in undetermined cases 99/169 (58.6%) ( p < 0.05). This fact suggests limited diagnostic efficiency. Deaths caused by infectious agents were higher than those related to noninfectious agents 49/169 (29.0%)( p < 0.05). Although no statistical differences were found between the proportions of undetermined cases with and without histopathological lesions ( p < 0.05), 44/99 (44.4%) of those cases had microscopic findings suggestive of an infectious disease.
Table 1. Diagnostic of neonatal mortality in 169 calves.

No significant differences were observed when proportions of determined and undetermined causes in beef and dairy calves were considered ( p > 0.05). Similarly, no significant differences were observed in the proportions of determined and undetermined causes on days when neonatal mortality was recorded ( p > 0.05).
Most common isolated agents were E. coli following by Brucella abortus, Arcanobacterium pyogenes and Salmonella spp . ( Table 1). In all septicemic cases due to E. coli, bacteria were isolated from several tissues. Similarly, septicemic forms caused by other bacteria isolated from multiple tissues included Nocardia spp. (lungs and liver), Pseudomonas aeruginosa (spleen, lymph node and gall), Salmonella spp. (intestine, spleen, mesenteric lymph node, gall bladder and synovia), and Streptococcus spp. (synovia). Dual or multiple infections due to several agents were recorded in 6 cases (Table 1). In addition, miscellaneous agents were frequently isolated in omphalophlebitis cases. BVDV was isolated in 2 (1.2%) cases and bovine rotavirus was identified in 5 (2.9%) cases, 2 of which E. coli.
Congenital abnormalities were the most common noninfectious etiology. These included spastic syndrome and paresis (1 case), cerebellar hypoplasia (2 cases), palatoschisis (2 cases), palatoschisis associated with hydranencephaly (1 case), hydrocephalus (2 cases), heart septal defects (2 cases), segmental aplasia of intestine (1 case) and hypoplasia of kidney (1 case).
DISCUSSION
Data of this study revealed that beef calves had died earlier than dairy calves. Although beef calves remain with their dams after birth and dairy calves are fed with milk or lacteal substitutes and concentrate nutriments, (11, 15) differences in mortality age could possibly be attributed to the differences in the management practices employed by beef and dairy farms in Argentina (i.e. human care to dairy calves prolonging their days of life). Similarly, in another study performed on beef calves, 75% of 178 calves died by day 2 postpartum (8). Different diseases or syndromes could be acting according to the type of production system. For instance, in the present work, B. abortus and congenital abnormalities (18 cases in total, Table 1) were only detected in beef calves. These etiologies caused death on the first days after birth. Nevertheless, although a higher number of dead calves were submitted during days 1 to 4, proportions of calves with determined and undetermined causes on days 1 to 4 compared with those on days 5 to 7, had similar distribution.
The high number of male beef calves (usually heavier and larger than females) submitted for diagnosis could be speculatively explained by dystocia problems as mentioned before (22). However, no gross lesions related to dystocia (edema in head, neck, tongue or forelimbs) were observed in the present work.
Data obtained in this study, where diagnoses on 70 (29%) cases corresponded to infectious agents, contrast with those reported by other authors (1). They considered that the death of 78% of 47 calves had been due to infections. However, the same authors attributed death by infectious disease to those specimens with histopathological changes compatible with presence of infectious agent but without isolation (1). Had calves with microscopic inflammatory lesions been considered in this work, our percentage of dead calves due to infectious causes would have reached 67.5% (Table 1). In the present study, histopathological examinations of tissues from undetermined cases showed that 26% of them had lesions which suggested an infectious origin. Using conventional methods, the final diagnosis rate in natural cases of bovine neonatal mortality is usually low (12, 18). The low sensitivity of all diagnostic tests used in this work plus a brief clinical history and, probably the most important, an extensive calf autolysis, could explain the poor final diagnosis rate. Moreover, the time between the moment of calf death and sampling is usually too long, which has a negative effect on the identification of the microorganisms involved. Another explanation for this high number of undetermined causes is that samples were not systematically taken from all specimens. This is due to several facts, namely, private practitioners usually demand specific tests, the farmer's interest in reducing the cost of the analysis or the previous diagnosis of a specific disease in the herd. This sampling methodology should be modified in future works.
As stated in other studies, our data also indicates that neonatal mortality due to bacteria was the most prevalent among the infectious agents (1, 2). Bacterial organisms associated with environmental, nutritional factors or poor husbandry can be causes of neonatal mortality (4, 5). Moreover, dystocia, stillbirth and hypothermia could increase the calf's susceptibility to infection by a wide variety of organisms during the first week of life (19, 41). Since multiple infections due to several bacteria, alone or associated with viruses, were recorded, any or all of these factors could have contributed to calf mortality in this study. Unfortunately, colostrum intake information was not recorded in this work.
E. coli produces enterotoxic and septicemic colibacillosis in young calves (27). In this study, septicemia was the most prevalent form and histopathological lesions were compatible with previous reports (1, 36). Although characterization of E. coli strains was not attempted, the high frequency of mortality and the presence of severe suppurative microscopic lesions in multiple tissues suggest high virulence in these isolated organisms. Although differences in management practices are employed in beef and dairy herds in Argentina (11, 15), this bacterium similarly affects calves from both types of herds. (data not shown).
To date, bovine brucellosis remains present in Argentina, and the disease is more frequent in beef herds (14). In the present study, B. abortus isolations were only obtained from beef newborn calves (data not shown), and could have been the result of congenital infection or infected colostrum intake. Unfortunately, this aspect was not clarified in this study.
The isolation of Salmonella spp. in this work suggests a role of this bacterium as etiological agent of bovine neonatal mortality; however, its frequency is generally low (7). This agent has been involved in bovine neonatal mortality and its high prevalence has been reported in other work (23). The fact that S. newport and S. enterica were isolated from gall bladder, spleen, intestine and feces (data not shown) in cases with presence of histopathological lesions in several tissues demonstrates the high virulence of this species (23).
Leptospirosis is usually associated with abortions, but premature weak and full-term calves may also be produced (29,39). In the present study, leptospires were only evidenced by DFAT in 1 case where non-suppurative multifocal nephritis, interstitial pneumonia and thyroiditis were also seen. Neonatal mortality associated with Leptospira infection may most frequently occur in the Humid Pampa; however, not only is isolation of Leptospira spp. difficult but also available diagnostic techniques have low (29).
C. perfringens type C enterotoxemia produce severe acute hemorrhagic enteritis in 1 to 10 day-calves (40). Unfortunately, this agent was not screened in this work.
Opportunistic bacteria can produce septicemia and neonatal mortality in calves (37, 38). In the present study, Actinomyces spp., Klebsiella spp., Streptococcus spp ., Nocardia spp ., Pseudomonas spp. and other miscellaneous bacteria were involved in neonatal mortality. Despite the fact that Staphylococcus dysgalactiae and Moraxella nonliquefaciens have not been commonly reported to be the cause of calf death, they were always isolated in pure culture and associated with the presence of histopathological lesions (Table 1). Factors such as strain virulence associated with poor hygiene and insufficient colostrum intake had probably provided the opportunity for these bacteria to reach several tissues by hematogenous route from the point of infection (6). Unfortunately, these factors were not established in the present work. It could be probable that the histopathological lesions found had been caused by other agent and the isolated bacteria had been contaminants.
One of the major causes of neonatal calf mortality and morbidity is diarrhea (8, 27). Although the causes of this syndrome could have both infectious or non-infectious origin (10), the presentation of diarrhea in the present work (data not shown) was only associated with infectious agents (diarrheagenic and septicemic forms of E. coli infection, rotavirus, Salmonella and BVDV infections (Table 1).
BVDV has high prevalence in Argentinean bovine herds (32). However, neonatal mortality due to BVDV was low (2 cases) in line with the results reported by others (1). Viral isolation is difficult due to poor viability of BVDV in autolyzed specimens submitted for diagnosis (30). Unfortunately, the origin of infection with DVBV in these 2 cases could not be clarified in the present study. However, infections causing death in calves are commonly associated with congenital transmission rather than with postnatal exposure (30).
Rotavirus is a common viral agent identified as the cause of neonatal diarrhea in Argentinean calves (31). However, studies indicate that mortality due to infection with rotavirus alone is very low (9), which is coincident with our records. Generally, rotavirus and bacteria act in synergic form in cases of enteritis (21) as was found out in this work (Table 1).
Other agents such as BHV and Cryptosporidium parvum involved in neonatal bovine morbidity and mortality (1, 12, 27) were not identified in the present work. Nevertheless, both agents have been previously recorded in this area (32, unpublished data).
In this work, congenital defects were found in non-viable calves. Environmental, genetic factors, teratogenic plants and infectious agents can be causes of abnormalities during bovine gestation (28). Usually, the origin of these congenital abnormalities is difficult to determine (33). However, in this study some lesions (cerebellar hypoplasia, palatoschisis, hydranencephaly) were suggestive of BVDV infection. Antecedents of Conium maculatum ingestion were recorded in 2 cases where congenital defects (palatoschisis) were seen (data not shown).
The data given in this report provide useful information to beef and dairy producers and veterinarians. Many calf deaths could be prevented if producers and veterinarians were more aware of the cause of calf mortality and their relative importance.
Although standard culture and histopathological techniques were successfully used for identifying some pathogens, the protocols and methodology should be improved in order to characterize the causes of bovine neonatal mortality. A higher etiological diagnosis could be achieved by the implementation of appropriate and modern techniques such as molecular tests. Such improvement in the diagnosis could help to establish strategic vaccination, adequate husbandry methods, management and biosafety.
Acknowledgements: We thank technicians at Animal Health Group, INTA Balcarce and resident veterinarians for technical support. We are also grateful to participating farmers and bovine practitioners for submitting specimens for diagnosis. Part of this work and the fellowship for Eleonora Morrell were granted by INTA and Agencia Nacional de Promoción Científica y Tecnológica, PICT 08-11139, Argentina.
1. Agerholm J, Basse A, Krogh H, Christensen K, Rønsholt L. Abortion and calf mortality in Danish cattle herds. Acta Vet Scand 1993; 34: 371-7. [ Links ]
2. Aldridge B, Garry F, Adams R. Neonatal septicemia in calves: 25 cases (1985-1990). J Am Vet Med Assoc 1993; 203: 1324-9. [ Links ]
3. Alton G, Jones L, Angus R, Verger J. Techniques for the Brucellosis Laboratory. INRA, 1988, p. 169-74. Paris, France. [ Links ]
4. Azzam S, Kinder J, Nielsen M, Werth L, Gregory K, Cundiff L, et al. Environmental effects on neonatal mortality of beef calves. J Anim Sci 1993; 71: 282-90. [ Links ]
5. Bakheit H, Greene H. Control of bovine neonatal diarrhoea by management techniques. Vet Rec 1981; 108: 455-8. [ Links ]
6. Baxter G. Umbilical masses in calves: diagnosis, treatment, and complications. Compend Contin Educ Vet 1989; 11: 505-13. [ Links ]
7. Bellinzoni R, Blackhall J, Terzolo H, Moreira A, Auza N, Mattion N, et al. Microbiology of diarrhoea in young beef and dairy calves in Argentina. Rev Arg Microbiol 1990; 22: 130-7. [ Links ]
8. Bellows R, Patterson D, Burfening P, Phelps D. Occurrence of neonatal and postnatal mortality in range beef cattle. II. Factors contributing to calf death. Theriogenology 1987; 28: 573-86. [ Links ]
9. Bezek D. Rotavirus enteritis in food animals. Compend Contin Educ Vet 1994; 16: 391-405. [ Links ]
10. Boland W, Cortese V, Steffen D. Interactions between vaccination, failure of passive transfer, and diarrhea in beef calves. Agri-Prac Imm 1995; 16: 25-8. [ Links ]
11. Buelink D, Schaller A, Labriola S. Principales Cuencas Lecheras Argentinas. Secretaría de Agricultura, Pesca y Alimentación, 1996, Suplemento Técnico 122, p. 1-48. Buenos Aires, Argentina. [ Links ]
12. Cain D, Dennis S. Perinatal calf mortality. Agri Pract 1987; 8: 11-6. [ Links ]
13. Campero C, Odriozola E, Odeón A, Casaro A. The causes of abortion and death occurring in calves during the first week of life in the south-east of Buenos Aires province, Argentina. Proceedings of the VII International Symposium of Veterinary Laboratory Diagnosticians, 1994, p. 104, Buenos Aires, Argentina. [ Links ]
14. Campero C, Moore D, Odeón A, Cipolla A, Odriozola E. Aetiology of bovine abortion in Argentina. Vet Res Commun 2003; 27: 359-69 . [ Links ]
15. Carrillo J, Schiersmann G. Beef cattle production in the temperate zone of South America (Argentina and Uruguay). In: Jarrige R, Béranger C, editors. Beef cattle Production. Amsterdam, World Animal Science Press, 1992, p. 259-69. [ Links ]
16. Cobo E, Cipolla A, Morsella C, Cano D, Campero C. Effect of two comercial vaccines to Campylobacter fetus subespecies on heifers naturally challenged. J Vet Med B 2003; 50: 75-80. [ Links ]
17. Costantini V, Parreño V, Combessies G, Bardón J, Leunda M, Saif L, et al. Diagnosis and antigenic characterization of group A bovine rotaviruses in Argentina, 1994 to 1999. 80th Annual Meeting of the Conference Research Workers in Animal Diseases, 1999, Abstract 224, p. 56, Chicago, USA. [ Links ]
18. Dennis S. Investigating perinatal calf mortality. Animal Meeting Society Theriogenology. 1980, Abstract 122, p.150, Omaha, USA. [ Links ]
19. Duhamel G, Osburn B. Neonatal immunity in cattle. Bovine Prac 1984; 19: 71-8. [ Links ]
20. Ellis W, O'Brien J, Nelly S, Ferguson H, Hanna J. Bovine leptospirosis: Microbiological and serological findings in aborted fetuses. Vet Rec 1982; 110: 147-50. [ Links ]
21. Hess R, Bachmann P, Baljer G, Mayr A, Pospischil A, Schmid G. Synergism in experimental mixed infections of newborn colostrum-deprived calves with bovine rotavirus and enterotoxigenic Escherichia coli (ETEC). Zbl Vet Med B 1984; 31: 585-96. [ Links ]
22. Holland M, Odde K. Factors affecting calf birth weight: a review. Theriogenology 1992; 38: 769-98. [ Links ]
23. House J, Smith B. Salmonella current concepts. Bovine Prac 1992; 30: 28-32. [ Links ]
24. Hubbert W. Committee on bovine reproductive nomenclature. Recommendations for standardizing bovine reproductive terms. Cornell Vet 1972; 62: 216-37. [ Links ]
25. Jones T, Glesier C. Veterinary Necropsy Procedures. En Jones TC, Gleiser CA, editors. J.B. Manual of Veterinary Necropsy Procedures. Philadelphia PA, JB Lippincott Co Press, 1954, p. 1- 136. [ Links ]
26. Kasari T. Weakness in the newborn calf. Vet Clin North Am Food Anim Pract 1994; 10: 167-80. [ Links ]
27. Khan A, Khan M. Aetiopatology of neonatal calf mortality. J Islamic Ac Sci 1991; 4: 159-65. [ Links ]
28. Leipold H, Dennis S. Congenital defects affecting bovine reproduction. In: Morrow DA, editor. Current Therapy in Theriogenology. Philadelphia, WB Saunders Press, 1986, p. 177-99. [ Links ]
29. Levett P. Leptospirosis. Clin Microbiol Rev 2001; 14: 296-326. [ Links ]
30. Nettleton P, Entrican G. Ruminant pestiviruses. Br Vet J 1995; 151: 615-42. [ Links ]
31. Odeón A, Casaro A, Villar J, Terzolo H. Diarrea neonatal de los terneros en el sudeste de la provincia de Buenos Aires (Argentina): Aspectos epidemiológicos, etiológicos, inmunológicos y patológicos. Rev Argent Prod Anim 1981; 1: 51. [ Links ]
32. Odeón A, Späth E, Paloma E, Leunda M, Fernández Sainz I, Pérez S, et al. Prevalencia de anticuerpos al virus de diarrea viral bovina, herpesvirus bovino y virus sincicial respiratorio bovino en Argentina. Rev Med Vet 2000; 82: 216-20. [ Links ]
33. Rousseaux C. Congenital defects as a cause of perinatal mortality of beef calves. Vet Clin North Am Food Anim Pract 1994; 10: 35-51. [ Links ]
34. Saif L, Smith K. Enteric viral infections of calves and passive immunity. J Dairy Sci 1985; 68: 206-28. [ Links ]
35. SAS Institute, Inc. SAS/STAT 1987, Guide for personal computers, Version 6 Edition. Cary, NC. [ Links ]
36. Seimiya Y, Ohshima K, Itoh H, Ogasawara N, Okutomo M, Tanaka S. Central nervous system lesions due to E. coli infection in neonatal calves. J Vet Med Sci 1992a; 54: 767-8. [ Links ]
37. Seimiya Y, Ohshima K, Itoh H, Ogasawara N, Okutomo M, Tanaka S. Clinicopathology of meningoventriculitis due to Streptococcus bovis infection in neonatal calves. J Vet Med Sci 1992b; 54: 871-4. [ Links ]
38. Seimiya Y, Ohshima K, Itoh H, Murakami R, Haritani M. A case of neonatal calf with meningitis associated with Klebsiella oxytoca infection. J Vet Med Sci 1993; 55: 141-3. [ Links ]
39. Smyth J, Fitzpatrick D, Ellis W. Stillbirth/perinatal weak calf syndrome: a study of calves infected with Leptospira. Vet Rec 1999; 145: 539-42. [ Links ]
40. Wikse S, Kinsel M, Field R, Holland P. Investigating perinatal calf mortality in beef herds. Vet Clin North Am Food Anim Pract 1994; 10: 147-66. [ Links ]
41. Wittum T, Dalman M, Odde K, Mortimer R, King M. Causes and cost of calf mortality in Colorado beef herds participating in the National Animal Health Monitoring System. J Am Vet Med Assoc 1993; 203: 232-6. [ Links ]
Recibido: 23/10/07
Aceptado: 26/05/08














