Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista argentina de microbiología
versión impresa ISSN 0325-7541versión On-line ISSN 1851-7617
Rev. argent. microbiol. v.41 n.1 Ciudad Autónoma de Buenos Aires ene./mar. 2009
Alterations in growth and branching of Neurospora crassa caused by sub-inhibitory concentrations of antifungal agents
1Departamento de Ciências Farmacêuticas, Faculdade de Ciências Farmacêuticas de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, 14040-903, São Paulo, Brazil;
2Gerência Geral de Medicamentos, Agência Nacional de Vigilância Sanitária, Ministério da Saúde, Brasília, 70770-502, Distrito Federal, Brazil.
*Correspondence. E-mail address: susaid@usp.br
ABSTRACT
Six antifungal agents at subinhibitory concentrations were used for investigating their ability to affect the growth and branching in Neurospora crassa. Among the antifungals herein used, the azole agent ketoconazole at 0.5 μg/ml inhibited radial growth more than fluconazole at 5.0 μg/ml while amphotericin B at 0.05 μg/ml was more effective than nystatin at 0.05 μg/ml. Morphological alterations in hyphae were observed in the presence of griseofulvin, ketoconazole and terbinafine at the established concentrations. The antifungal agents were more effective on vegetative growth than on conidial germination. Terbinafine markedly reduced growth unit length (GU) by 54.89%, and caused mycelia to become hyperbranched. In all cases, there was a high correlation between hyphal length and number of tips (r > 0.9). All our results showed highly significant differences by ANOVA, (p < 0.001, α = 0.05). Considering that the hyphal tip is the main interface between the fungus and its environment /through which enzymes and toxins are secreted and nutrients absorbed, it would not be desirable to obtain a hyperbranched mycelia with inefficient doses of antifungal drugs.
Key words: Antifungal drugs; Apical growth; Neurospora crassa.
RESUMEN
Alteraciones de crecimiento y ramificación en Neurospora crassa provocadas por concentraciones subinhibitorias de agentes antimicóticos. Se investigó el efecto de seis agentes antimicóticos en concentraciones subinhibitorias sobre el crecimiento y la ramificación en Neurospora crassa. El agente azólico ketoconazol a la concentración de 0,5 μg/ml inhibió el crecimiento radial más que el fluconazol a 5,0 μg/ml, y la anfotericina B a 0,05 μg/ ml fue más eficiente que 0,05 μg/ml de nistatina, entre los agentes poliénicos usados. En presencia de griseofulvina, ketoconazol y terbinafina a las concentraciones establecidas se observaron alteraciones morfológicas en las hifas. Los agentes antimicóticos fueron más eficientes sobre el crecimiento vegetativo que sobre la germinación conidial. La terbinafina redujo marcadamente (54,89%) la longitud de la unidad de crecimiento y provocó la hiperramificación del micelio. En todos los casos, existió gran correlación entre la longitud y el número de ápices de las hifas (r > 0,9). Todos los resultados mostraron diferencias altamente significativas de acuerdo con ANOVA (p < 0,001, α = 0,05). Considerando que el ápice de la hifa es la principal interfase entre el hongo y su ambiente, a través de la cual las enzimas y las toxinas son secretadas y los nutrientes son absorbidos, un micelio hiperramificado resultante de dosis ineficientes de agentes antimicóticos sería perjudicial.
Palabras clave: Drogas antimicóticas; Crecimiento apical; Neurospora crassa.
INTRODUCTION
Due to several correlated factors like the emergence of AIDS and other highly infectious diseases, organ transplantation and cancer chemotherapy, the nosocomial immunocomprised population has noticeably increased over the last decades, bringing along the spectre of opportunistic fungal infections (5, 21, 25). It is important to call attention to the limited number of effective antifungal compounds available and the need to develop new clinical options (3, 9). Fungi are eukaryotic organisms that share a large set of potential drug targets with their hosts, hindering the development of new agents (21). In spite of this, new azoles (voriconazole and posaconazole), a class of echinocandins, especially caspofungin and anidulafungin, have recently been examined (8, 15, 24).
The aim of the present work was to study the effects of subinhibitory concentrations of six antifungal drugs on different phases of the development of N. crassa, used as fungal model, by analyzing changes in conidial germination, vegetative growth and morphology of hyphal branching.
MATERIALS AND METHODS
Microorganism
Neurospora crassa wild type 74OR 8-1a (ATCC) kept on slants of Vogel's Minimum Medium (VMM) supplemented with 2% (w/v) glucose and stored at 4 oC, was used throughout this work. For the experiments, fungus was grown in this medium at 30± 1°C for 3 days, and at room temperature for 4 days.
Antifungal drugs
Sub-lethal concentrations of six antifungal agents from four classes of antibiotics having different mechanisms of action were tested. Griseofulvin, a carbamate, exerts its fungistatic action by inhibiting cell division, leading to the disruption of the mitotic spindle structure (22). Fluconazole and ketoconazole are azole agents that decrease ergosterol synthesis by inhibiting cytochrome P450 enzymes (16). Terbinafine, an agent from the allylamines class, acts by inhibiting the enzymes responsible for squalene conversion (17). Polyenic macrolides (nystatin and amphotericin B) have in common a mechanism of action by means of which they selectively bind to ergosterol (7). In earlier experiments, different concentrations of antifungals have been tested in order to establish those that would allow a certain fungus growth degree. Lethal concentrations of antifungals would not fulfill the aims of this study. It is worth pointing out that the excipient used, usually lactose and/or magnesium stearate contained in the tablets or capsules wherefrom the employed antifungals were extracted, are innocuous for man. Tablets or capsules were pulverized and dissolved in dimethylsulphoxide [DMSO 99.5% (v/v)] to yield drug solutions at the established concentrations, after adequate dilution. Samples were aseptically added to the VMM at 45 °C. The final medium DMSO concentration was adjusted to a maximum of 0.5% (v/v). Controls contained the same final DMSO concentration, but no antifungal drug was added.
Evaluation of antifungals on fungal growth and morphology
To evaluate the effect of antifungal drugs on N. crassa growth, 7-day old conidia were inoculated on VMM containing the antifungals, in Petri dishes and incubated at 30 ± 1 °C for 24 h. Growth was followed by measuring colony radii after 7; 15; 20 and 24 h in three replicates for each concentration. Fungus growth rate was determined using 50.00 cm race tubes containing VMM supplemented or not with each antifungal. Media at the end of the race tube were inoculated with conidia. Mycelia lengths were daily measured. Growth rate was calculated from averages obtained after a 24-h interval. Growth Units (GUs) were determined according to Trinci's technique (23): approximately 50 conidia were inoculated on VMM in Petri dishes, spread and incubated at 30 °C for 15 h. Photographs were taken with a Zeiss Stemi SV11 stereomicroscope under transmitted light using T-MAX 400 film. Total hyphal lengths and number of branches per hyphal length were measured on the enlarged photographic prints. GU values at each experimental condition were calculated from the quotient between total length and number of branches.
Evaluation on conidial germination and vegetative growth
Approximately 100 conidia were inoculated on dialysis membranes, overlaying VMM with antifungals, and incubated for 5 h at 30 oC. The total numbers of conidia, as well as the amounts germinated were evaluated under a Zeiss Axioskop microscope. To assess the effect of drugs on vegetative hyphae, conidia were allowed to grow for 24 h on VMM. Mature hyphae were then sectioned about 1.0 cm below the apex, allowing to rest on the agar for 1 h to achieve osmotic stabilization. Next, the pieces of dialysis membranes covered with hyphae, were transplanted onto VMM supplemented with different antifungal drug concentrations and incubated for an additional 24 hours.
Statistical Analysis
ANOVA and Student's t tests for significance were performed at the level of α = 0.05. Correlation between hyphal length and number of tips (GU) was determined by regression analysis.
RESULTS
Radial growth
In previous experiments, several concentrations of each antifungal had been tested to determine the sublethal concentration. We observed a significant colony radial growth inhibition compared to controls, but less than 50% (data not shown). A concentration was established for each drug according to its effects (5.00 μg/ml griseofulvin; 5.00 μg/ml fluconazole; 0.50 μg/ml ketoconazole; 0.25 μg/ml terbinafine; 0.05 μg/ml nystatin; 0.05 μg/ml amphotericin B). At equal concentration, fluconazole caused an inhibition approximately 4-fold higher than that produced by griseofulvin (Table 1). At one-half the concentration of ketoconazole, the inhibition by terbinafine resulted in a 2-fold increase. Among the polyene antifungals, amphotericin B led to a 33% growth inhibition, whereas, nystatin at the same concentration, did not result in inhibition. Neither DMSO used to prepare the test solutions nor the pharmaceutical excipients presented significantly inhibited growth at the doses of antifungals employed (Student's t, p < 0.001, α = 0.05).
Table 1. Standard concentration of antifungals and inhibitory effect on colony radius.
Alterations in hyphal morphology
It is known that in its normal growth, Neurospora crassa displays highly polarized and regularly spaced branches emerging from a main hypha (Figure 1D). In the presence of griseofulvin (Figure 1A), the branches became curled denoting a loss of the polarized growth. Supplementation of VMM with ketoconazole resulted in shorter and bud-like branches (Figure 1B); terbinafine induced unusual multipolar germination of conidia leading to short hyphae (Figure 1C). No alterations on hyphal morphology were detected in nystatin, amphotericin B and fluconazole presence.
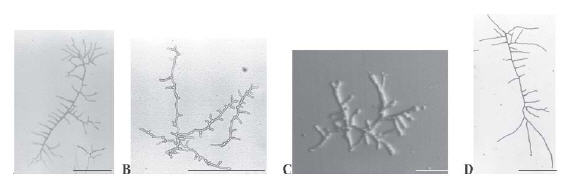
Figure 1. Antifungal effect on morphology and branching pattern of N. crassa hyphae. Micrographs of single hyphae grown on supplemented (A, griseofulvin; B, ketoconazole; and C, terbinafine) or non-supplemented (D) VMM were taken after 15 h of incubation at 30 °C. Bars, 100 μm (A, B e D) and 500 μm (C).
Percentages of conidial germination and inhibition of vegetative growth
All drugs caused a reduction of approximately 30 ~ 40% of conidial germination after 5 h incubation, with the exception of terbinafine, in whose presence less than 20% of the total conidia inoculum, germinated (Figure 2). Terbinafine was more effective on conidial germination than on inhibiting vegetative growth (80% against 50%, respectively). The inhibitor effect of griseofulvin, fluconazole and ketoconazole was stronger on vegetative growth than on conidial germination. Polyene drugs did not significantly inhibit vegetative growth.

Figure 2. Effect of antifungals on conidial germination and vegetative growth of N. crassa. Seven-day old conidia or 24-h-old hyphae transplanted from non-supplemented VMM, were inoculated onto antifungal-supplemented media, overlaid with dialysis membranes, and incubated at 30 °C for 5 h for conidial germination (white bar) and 24 h for vegetative growth (solid bar). Griseofulvin (grs), fluconazole (flz), ketoconazole (ktz), terbinafine (terb), nystatin (nys), and amphotericin B (AmB).
Effect on growth rate
Terbinafine caused a strong reduction of N. crassa growth rate (Table 2), whereas none of the polyene antifungals showed this effect to a significant extent (ANOVA, α = 0.05). The other agents tested only exhibited moderate inhibition effects. Under the conditions employed, an initial 24-hour adaptation period post inoculation was shown, followed by a constant growth rate. Adaptation to terbinafine required approximately 48 h.
Table 2. Growth rates of N. crassa in drug-supplemented media.

Alterations in the hyphal growth unit
The hyphal GU is the ratio between the length of a given hypha and its number of tips or branches. The total length and number of tips per hypha were determined from digitally enhanced micrographs of single hypha. An extremely significant level of correlation (r) was obtained at all conditions examined (Table 3); and run tests indicated that a linear model was followed in each case (2). ANOVA (α=0.05) of these results indicated a highly significant difference at different conditions. Dunnett's multiple comparison test showed that griseofulvin, fluconazole and ketoconazole did not significantly altered GU, while terbinafine caused total lengths to be reduced by more than 50%. Polyene antifungals increased GU values by 45 ~ 55% (Figure 3).
Table 3. Effect of antifungals on the GU of N. crassa.
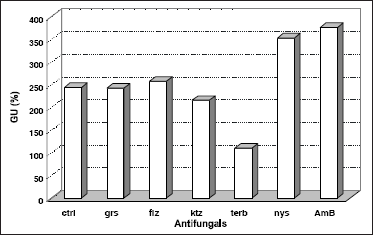
Figure 3. Effects of antifungals on Growth Unit (GU) of N. crassa. Parameters were obtained from the ratios between total length of a given hypha and its respective number of tips, shown on digitally enhanced micrographs of at least 8 determinations. Controls (ctrl) contained no antifungal; standard errors remained below 10%. Griseofulvin (grs), fluconazole (flz), ketoconazole (ktz), terbinafine (terb), nystatin (nys), and amphotericin B (AmB).
DISCUSSION
It has been reported that DMSO concentrations greater than 2.0% may inhibit growth and disrupt Neurospora crassa's apical actin cap (4, 11). The concentration used in the present work (0.5% v/v) caused none of these effects when radial growth was evaluated. A direct relationship between the antimycotic concentration and radial growth inhibition was observed for all drugs studied. However, agents acting on similar metabolic fungal pathways affected them to a different extent. For instance, the same concentration of amphotericin B and nystatin (0.05 μg/ ml) caused a 33.54% inhibition and no inhibition, respectively. Drugs acting on the same molecular targets, like fluconazole and ketoconazole, respectively inhibited fungal growth in different ways. Such differences may be explained by the drug's structural variety leading to different binding affinities to affected enzymes. Terbinafine's inhibition of squalene epoxidase has been proven to be more effective than azole's action on 14α-demethylase (25). It is also known that fungi present distinct physiological features according to their various developmental stages (18). Therefore, in this study, the antimycotic effect was evaluated during both conidial germination and vegetative growth phases and as a result, a variety of effects were observed. Thus, although unable to cause a significant inhibitory effect during vegetative growth, macrolides reduced conidia germination by approximately 30%. Although azole drugs are known to act on identical molecular targets, they affected fungal growth differently according to their phase. Fluconazole reduced vegetative growth by 52.26%, but ketoconazole caused only a 28.66% inhibition. In turn, ketoconazole reduced conidial germination by 42.20% while fluconazole reduced this parameter by 32.74%. Structure-activity relationships could account for such differences (20). Irrespective of the growth phase, terbinafine proved to be the most effective agent of all those studied, as it decreased vegetative growth by 53.5% and conidial germination by 79.58%. With the exception of nystatin and amphoterin B, antifungals added to VMM, on average, significantly reduced the growth rate between 8.58% for fluconazole and 65.45% for terbinafine. It has been previously established for a variety of fungal species, that different growth conditions or the presence of inhibitors can lead to alterations in hyphae morphology, growth and branching patterns (23). Determining the hyphal growth unit could bring about new data concerning these alterations. The present results show that griseofulvin, fluconazole and ketoconazole, kept the growth unit and hyphal density unaltered relative to controls. On the other hand, terbinafine significantly reduced the growth unit length by over 50%. This could be explained as a result of the higher number of branches per length of hypha (i.e. the number of branches in the terbinafine-supplemented medium could practically be doubled with respect to the controls).
Hyphae morphology on non-supplemented VMM showed polarized growth and regularly spaced branches, in agreement with previous reports (1). Under the effect of griseofulvin, hyphae became rugged and curled. This phenotype is similar to that of the N. crassa dynein-deficient ropy mutant (19). Fluconazole caused slight increase in mycelium density and branches emerged in irregular fashion from the main hypha. Also, at closer examination, apex frequently showed a dichotomic pattern very similar to that observed in hyphae submitted to cold shock (12) or in the presence of the calcium channel blocker, verapamil (6). The incorporation of ergosterol precursors into the plasma membrane may also lead to a barrier of altered fluidity, reflecting effect on the function of calciumdependent ATPases and other integrating proteins (13). The hyperbranched growth pattern observed under the effect of terbinafine closely resembled that of spray and frost N. crassa mutants (6). It is generally accepted that for filamentous fungi, life is at the tip (10), since the main biological processes such as growth, reproduction, morphogenesis, substrate absorption, environment recognition, and protein secretion occur in the apical region. It is to be noted that previous reports had shown that protein secretion in filamentous fungi is restricted to the growing tips and that conditions that increase growing surface lead to an increase in the amount of protein secretion (14).
In this work, we demonstrated that at least two of the antimycotics tested, induced morphological alterations. Griseofulvin disorganized polarized growth, while terbinafine led to hyperbranching. Considering that increased branching could result in increased active growth area, it may be speculated that for pathogenic fungi this could be translated into increased pathogenicity. Misuse of medicines, especially of antibiotic agents, is a well known clinical occurrence and the main cause of bacterial (and, more recently demonstrated) fungal resistance. Early interruption of treatment, often times prior clinical cure, can result in the selection of resistant strains. Eventually, plasma levels of these drugs could decrease to levels similar to those shown in our work to have caused hyperbranching.
In conclusion, a potentially more resistant strain may be induced to increasing branching that in turn, may lead to a higher level of protein and toxin secretion.
Acknowledgements: This work was supported by grants from Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) no 97/03619-7 and is part of a thesis submitted by Rogério Coutinho Pereira to Faculdade de Ciências Farmacêuticas de Ribeirão Preto, Universidade de São Paulo, in partial fulfillment of the requeriments for the Master's degree. RCP received a master fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). We are thankful to M. A. S. C. Chellegatti for typing the manuscript.
1. Barja F, Thi B, Turian B. Localization of actin and characterization of its isoforms in the hyphae of Neurospora crassa. FEMS Microbiol Lett 1991; 77: 19-24. [ Links ]
2. Bolton S. Analysis of variance. In: Bolton S, editor. Pharmaceutical Statistics: Practical and Clinical Applications. New York, Marcel Dekker, 1997, vol. 737, p. 265-325. [ Links ]
3. Cassone A. Cell wall of pathogenic yeasts and implications for antimycotic therapy. Drug Exp Clin Res 1986; 12: 635-43. [ Links ]
4. Chalmers JH. Toxicity of antibiotics and other drugs to Neurospora. Neurospora Newsletter 1974; 21: 20-1. [ Links ]
5. Debono M, Gordee RS. Antibiotics that inhibit cell wall development. Annu Rev Microbiol 1994; 48: 471-97. [ Links ]
6. Dicker JW, Turian G. Calcium deficiencies and apical hyperbranching in wild-type and the frost and spray morphological mutants of Neurospora crassa. J Gen Microbiol 1990; 136: 1413-20. [ Links ]
7. Ellis D. Amphotericin B: spectrum and resistence. J Antimicrob Chemother 2002; 49: 7-10. [ Links ]
8. Falagas ME, Ntziora F, Betsi GI, Samonis G. Caspofugin for the treatment of fungal infections: a systematic review of randomized controlled trials. Int J Antimicrob Agents 2007; 29: 136-43. [ Links ]
9. Ghannoum MA, Rice LB. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev 1999; 12: 501-17. [ Links ]
10. Gooday GW, Gow NAB. Tip growth. In: Heath IB, editor. Tip Growth in Plant and Fungal Cells. San Diego, Academic Press, 1990, p. 31-58. [ Links ]
11. Jackson SL, Heath IB. Evidence that actin reinforces the extensible hyphal apex of the oomicete Saprolegnia ferax. Protoplasma 1990; 154: 66-70. [ Links ]
12. Kawano CY, Said S. Morphological alterations induced by cold shock in Neurospora crassa. J Basic Microbiol 2002; 42: 381-7. [ Links ]
13. Lee AG. How lipids interact with an intrinsic membrane protein: the case of the calcium pump. Biochim Biophys Acta 1998; 1376: 381-90. [ Links ]
14. Lee IH, Walline RG, Plamann M. Apolar growth of Neurospora crassa leads to increased secretion of extracellular proteins. Mol Microbiol 1998; 29: 209-18. [ Links ]
15. Letscher-Bru V, Herbrecht R. Caspofugin: the first representative of a new antifungal class. J Antimicrob Chemother 2003; 51: 513-21. [ Links ]
16. Odds FC. Resistance of yeasts to azole-derivative antifungals. J Antimicrob Chemother 1993; 31: 463-71. [ Links ]
17. Petranyi G, Meingassner JG, Mieth H. Antifungal activity of the allylamine derivative terbinafine in vitro. Antimicrob Agents Chemother 1987; 31: 1365-8. [ Links ]
18. Schmith JC, Brody S. Biochemical genetics of Neurospora crassa conidial germination. Bacteriol Rev 1976; 40: 1-41. [ Links ]
19. Seiler S, Plamann M, Schliwa M. Kinesin and dynein mutants provide novel insights into the roles of vesicle traffic during cell morphogenesis in Neurospora. Curr Biol 1999; 9: 779-85. [ Links ]
20. Staben C. Resistance to azole drugs in Neurospora crassa. Exp Mycol 1995; 19: 163-5. [ Links ]
21. Stephenson J. Investigators seeking new ways to stem rising tide of resistant fungi. JAMA 1997; 277: 1-10. [ Links ]
22. Tamaki K. Successful treatment of pigmented purpuric dermatosis with griseofulvin. British J Dermatol 1995; 132: 159-60. [ Links ]
23. Trinci APJ. The hyphal growth unit of wild type and spreading colonial mutants of Neurospora crassa. Arch Microbiol 1973; 91: 127-36. [ Links ]
24. Vazquez JA. Anidulafungin: a new echinocandin with a novel profile. Clin Ther 2005; 27: 657-73. [ Links ]
25. White TC, Marr KA, Bowden RA. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev 1998; 11: 382-402. [ Links ]
Recibido: 15/07/08
Aceptado: 23/12/08.














