Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Revista argentina de microbiología
versão impressa ISSN 0325-7541versão On-line ISSN 1851-7617
Rev. argent. microbiol. v.41 n.4 Ciudad Autónoma de Buenos Aires out./dez. 2009
ARTÍCULO ORIGINAL
Echinococcus granulosus: biological comparison of cattle isolates from endemic regions of Argentina and Spain
M. V. Andresiuk*1,2, F. Ponce Gordo3, C. Cuesta Bandera3, M. C. Elissondo1, 2, M. Dopchiz1, 2, G. Denegri1, 2
1Laboratorio de Zoonosis Parasitarias. Facultad de Ciencias Exactas y Naturales. Universidad Nacional de Mar del Plata. Funes 3350 (7600) Mar del Plata, Buenos Aires, Argentina;
2Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina;
3Departamento de Parasitología, Facultad de Farmacia, Universidad Complutense de Madrid, Madrid, España.
*Correspondence. E-mail: mandres@mdp.edu.ar
ABSTRACT
In the present study we have compared cattle isolates of Echinococcus granulosus from Argentina and Spain. The aim was to compare and determine if there exist phenotypic and genetic differences within E. granulosus cattle isolates between an endemic area of Spain (where the disease is mainly restricted to a sheep-dog cycle) and an endemic area of Argentina (where cattle are the most abundant intermediate hosts). The Spanish samples were previously identified as G1 genotype. The Argentinean samples were also identified as G1, but some variants were found for the cytochrome c oxidase-1 (CO1) and NADH dehydrogenase-1 (ND1) mitochondrial genes. When comparing the cyst features and the morphology of the larval rostellar hooks in both regions, some differences were found. The morphometric analyses of the larval rostellar hooks showed the existence of two distinct clearly separated groups (one corresponding to the Argentinean samples and the other to the Spanish ones). In conclusion, there are some genetic and phenotypic differences within E. granulosus cattle isolates from Argentina and Spain. Probably these differences, more important from an epidemiological point of view, are related to different steps in the disease control in both countries. Further studies involving other epidemiological, morphometric and molecular data, including other types of livestock, would contribute to clarify and expand the present work.
Key words: Echinococcus granulosus; Epidemiology; Morphology; Genotypes; Argentina; Spain.
RESUMEN
Echinococcus granulosus: comparación biológica de aislados de bovinos de regiones endémicas de Argentina y España. El objetivo del presente trabajo fue determinar si existen diferencias fenotípicas y genéticas entre los aislados de Echinococcus granulosus de origen bovino provenientes de dos regiones geográficas donde la hidatidosis es endémica, una de España (donde predomina el ciclo perro-oveja) y una de Argentina (donde el bovino es el hospedador intermediario más importante). Las muestras españolas fueron previamente identificadas como pertenecientes al genotipo G1. Las muestras argentinas también correspondían al genotipo G1, pero entre ellas se registraron algunas microvariantes de los genes mitocondriales citocromo c oxidasa-1 (CO1) y NADH deshidrogenasa- 1 (ND1). La comparación de las características de los quistes y de la morfología de los ganchos rostelares del metacestode mostró ciertas diferencias. En conclusión, existen algunas diferencias genéticas y fenotípicas entre los aislados de E. granulosus de Argentina y España. Probablemente estas diferencias, más importantes desde el punto de vista epidemiológico, podrían estar relacionadas con diferentes etapas en los programas de control de la enfermedad en los dos países. Estudios adicionales que involucren datos epidemiológicos, morfométricos y moleculares provenientes de otros tipos de ganado contribuirán a clarificar y ampliar la información aportada por este trabajo.
Palabras clave: Echinococcus granulosus; Epidemiología; Morfología; Genotipos; Argentina; España.
INTRODUCTION
Echinococcosis-hydatidosis is a cosmopolitan zoonosis caused by the cestode Echinococcus granulosus. This parasite shows great intraspecific variability in relation to its host specificity, epidemiology, morphology, biochemistry, physiology and genetics (39). Various methods based on morphology, physiology, biochemistry and immunology have been used to characterize the variants or strains of E. granulosus (1, 2, 31, 39). These strains were later identified as genotypes by molecular studies (G1 - G10) (7-9, 24, 35). Recently some authors have proposed a revision of the genus based on phylogenetic studies tending to re-categorize some genotypes to the species level (27, 34, 41). Several studies around the world that have been carried out to characterize the strains/genotypes of E. granulosus from a region or a country, have demonstrated the existence of genetic variation or sequence heterogeneity in mitochondrial DNA within some of the recognized genotypes G1 - G10 (21, 22, 29, 33). Haag et al. (19) gave the name of “haplotypes” to the mitochondrial sequences used to discriminate strains, and “variants”, to the haplotypes with minor genetic differences within a strain. Since then, different authors have described these mitochondrial variants or microvariants within E. granulosus (5, 6, 10, 32). Among the ten genotypes that have been described so far, the G1 genotype is the most widespread around the world infecting sheep, cattle, pig, goat, buffalo and humans. In general, cattle have been considered a poor suitable host for the G1 genotype, although some recent studies have demonstrated that cattle could play a role as a reservoir of the G1 genotype in some areas like Algeria and Tunisia, which is a serious risk for human health (4, 25, 40).
Although E. granulosus is widespread in the whole territory of Argentina and the hydatid disease is considered endemic in this country, the infection does not have a homogeneous geographical distribution. The contribution of each kind of livestock (cattle, pig, sheep, etc) in the disease transmission depends on the region: for example while sheep is the principal host in the Patagonian and Mesopotamian region, cattle is the prevalent one in the Pampa and northern regions of the country (14). Particularly in the south-eastern region of Buenos Aires province (located within the Pampa region), hydatidosis - echinococcosis is considered an important human and veterinary health problem, being cattle the main livestock involved in the transmission of this disease. (3, 14, 15, 16, 20)
In Spain, E. granulosus infects sheep, cattle, pig, goat and humans and several genotypes have been described. However, sheep is the main host involved in the transmission of the hydatid disease (11, 13, 18, 26, 30, 36).
The aim of the present study is to compare and determine if there exist phenotypic and genetic differences between E. granulosus isolates of cattle origin from an endemic central area of Spain (where the disease is mainly restricted to a sheep-dog cycle) and from an endemic area of Argentina (southern region of Buenos Aires province) where cattle are the most abundant intermediate hosts.
Material and methods
Origin and processing of samples
The hydatid cysts were obtained from liver and lungs of infected cattle. The Spanish samples were collected during the period 1990-1996 at slaughterhouses from the endemic central region of Spain (Autonomous Community of Madrid, Castilla - La Mancha, Castilla - León, Extremadura, Aragón and Navarra). The Argentine samples were collected between 2003 and 2004 at an abattoir located in the south-eastern region of Buenos Aires province. Each cyst was processed as an individual isolate. They were opened under sterile conditions, according to Smyth and Davies (38). For each cyst, the external appearance and macroscopical characteristics of the cyst wall, germinal membrane and hydatid fluid (general aspect, color and transparency, existence of calcified or degenerated areas and contamination) were recorded. For the purposes of this study, the cysts considered viable were those having membranes which did not appear upset or degenerate, white, without calcified, caseous or degenerated regions, not contaminated and with a transparent hydatid fluid. Those cysts which had protoscoleces were considered fertile. The vitality of protoscoleces (percentage of alive protoscoleces) was determined by their overall aspect (motility, flame cell activity, presence of calcareous corpuscles) and negative methylene blue staining. Viable and fertile cysts were further processed; germinal membranes were washed three times in sterile phosphate buffered saline (PBS) and stored at 4 °C (if to be processed within a short period of time) or at -20 °C until use. When hydatid sand was present, it was processed according to Smyth and Davies (38) and then stored in alcohol 70°-glycerine (1:1) at room temperature (for morphological studies) or in alcohol 70° at 4 °C or PBS at -20 °C (for genetic analyses).
Epidemiological data
The number and location of cysts, their size, viability and fertility were recorded for each geographical area under study. Comparisons between both regions were made using univariate techniques for all the recorded variables (Fisher's Exact Test, Mann Whitney and Student's t test) (39). Statistical significance was assessed at ρ ≤ 0.05. Mean intensity was calculated as the mean number of cysts/infected animals (39).
Morphological studies
The protoscoleces were processed according to Ponce Gordo and Cuesta Bandera (31). The number, shape and arrangement of rostellar hooks were recorded, and 5 variables were used for statistical analysis: the total number of hooks per rostellum (NUM), the blade length of large (LBL) and small (SBL) hooks, and the total length of large (LTL) and small (STL) hooks. All measurements were made by the same people (M.V.A. and F.P.G. for the Argentinean samples and F.P.G. for the Spanish samples). Comparisons between and within each region were made by using univariate and multivariate techniques (Principal Component Analyses) (31).
DNA analysis and sequencing
The identification of the Spanish isolates as belonging to the sheep strain/G1 genotype had been previously carried out by using biochemical and genetic analyses (13, 18, 26, 30, 36, 37). All the Spanish isolates presented 100% homology with the cytochrome c oxidase-1 (CO1) and NADH dehydrogenase-1 (ND1) sequences of the G1 genotype originally described by Bowles et al. (7) and Bowles and McManus (9) (GenBank Accession Numbers: M84661 and AJ237632).The genotype of the Argentinean isolates has been identified by using the same mitochondrial genes, following the same protocols and using the same PCR conditions as previously described by other authors (7, 9, 26). The gene segment considered was 366bp in length for CO1 (7) and 471bp in length for ND1 (9). The CO1 and ND1 fragments were purified using the QIAquick PCR Purification Kit (Qiagen). Their sequences were obtained in an automatic DNA sequencer (ABI PRISM Model 377 Perkin Elmer) and compared with the ND1 and CO1 sequences of the G1-G10 genotypes available at the NCBI database (28).
RESULTS
Epidemiological analysis
A total of 22 cattle were infected with E. granulosus in Argentina and 94 in Spain. The mean intensity of infection for Argentinean cattle was 18.55 (408 cysts) and 5.61 (527 cysts) for the Spanish ones. Whereas all Argentinean cattle presented viable cysts, only 10.64% (10) of the Spanish cattle presented them. The percentage of cattle with fertile cysts was clearly higher in Argentina (63.64%) than in Spain (6.38%). Percentage of cattle harboring viable and fertile cysts was significantly higher in Argentina (χ2 = 71.26, ρ < 0.00000 and χ2 = 40.95, ρ < 0.00000 respectively). Percentages of cattle with pulmonary or hepatic cysts were higher in Argentina (95.45% and 31.82) than in Spain (78.72% and 29.79%) but did not show statistically significant differences between both regions (χ2 = 3.36, ρ = 0.07 and χ2 = 0.03, ρ = 0.85 respectively). Only 9.09% (2) of Argentinean cattle presented cysts in other locations. Almost all the cysts were located in the liver or lungs, being the latter the preferred organ in both regions.
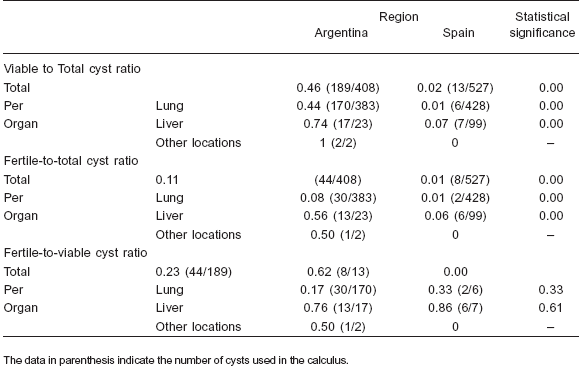
The total number of cysts, the number of viable and fertile cysts and the distribution of cysts by location are shown in Table 1. However, while the mean intensity of liver infection was similar in Argentinean and Spanish cattle, the mean intensity of lung infection was about four times higher in the Argentinean ones. The mean lung and liver intensity of viable and fertile cysts and the viable-tototal- cysts ratios were clearly higher in the Argentinean samples; however the total fertile-to-viable-cysts ratio was higher in the Spanish samples, although no differences were found in this ratio when lung and liver cysts were considered separately (Table 2).
Table 1. Characteristics of the different kinds of hydatid cysts (total and per organ) in cattle from Argentina and Spain. S.D.: standard deviation
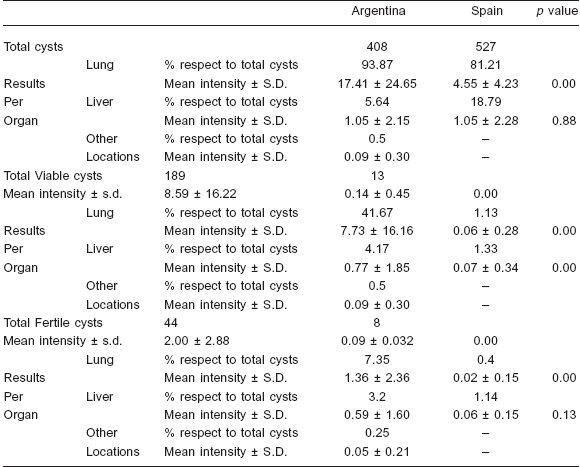
Table 2. Ratios (total and per organ) of viable and fertile cysts in cattle from Argentina and Spain
The size and the external aspect of the cysts were similar in both regions. While most of the cysts ranged from 3 to 4 cm in diameter for both regions, there were some up to 25 cm in diameter in the Argentinean samples. In all cases, the color of the germinative membrane was whitish to yellowish and it was usually thin, with a mucous aspect, and detached from the cyst wall. The hydatid sand was whitish in fertile cysts with protoscoleces and brood capsules in the Argentinean and Spanish samples. Few protoscoleces were present in the fertile Spanish samples with a vitality percentage (alive/not alive protoscoleces) not higher than 80%, whereas a great number of protoscoleces was present in the fertile Argentinean samples with a vitality percentage between 80 and 100%.
Morphological studies
The appearance of the larval rostellum in isolates from Argentina and Spain was similar and consisted of 2 rows of alternating large and small hooks. The comparisons between the measurements of the different variables for each of the groups (Argentina and Spain) were statistically analysed by the Student's t test. All of them but one (LTL) presented significant statistical differences (Table 3). Principal Components Analysis (PCA) was carried out with data from 25 isolates of Argentinean cattle and the 8 fertile isolates from Spain. Significant correlations were found, indicating that an increase in the number of hooks correlates with a longer blade in both the large and small hooks and with a shorter total length of the small hooks. The criterion considered for the number of factors to be extracted was the “greatest of the 75% variance rule” and the Scree test described by Cattell (12). Two factors were finally extracted, which represented nearly 78% of the variance. When isolates were plotted using both factors, two different groups became evident: one corresponding to the Argentinean isolates and the other to the Spanish ones (Figure 1).
Table 3. Rostellar-hook measurements of the protoscoleces of E. granulosus from hydatid cysts of cattle origin from Argentina and Spain. S.D: standard deviation
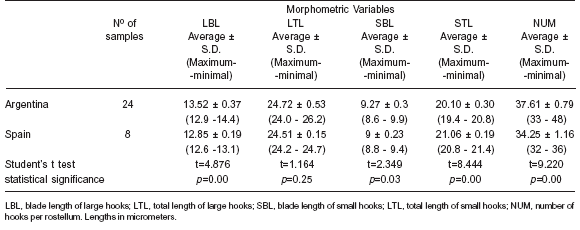
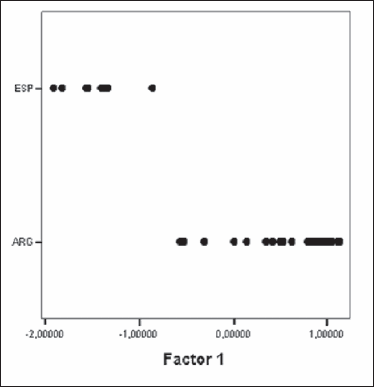
Figure 1. Plot of E. granulosus isolates from cattle of Argentina and Spain obtained by the Principal Component Analysis. ESP: Spain, ARG: Argentina.
Genotype identification
All Argentinean isolates (a total of 25) belonged to the G1 genotype, but different variants of the G1 haplotype originally described (GenBank accession no. M84661 and GenBank accession no. AJ237632) were identified for the CO1 and NADH1 mitochondrial genes in the present study. While some sequences were identical to the original one described for the CO1 gene (GenBank accession no. M84661), there were two other different variants in the CO1 sequence with transition mutations: one variant with a mutation at position 73 (CxT) (GenBank accession no. AF458871) and the other one with a mutation at position 111 (TxC) (GenBank accession no. AF458875). For the NADH1 gene only one sample was identical to the original one (GenBank accession no. AJ237632) and 3 other different variants were registered in the rest of the sequences: one of the variable sequences showed a transition mutation at position 282 (TxC) (GenBank accession no. AF297617), another one showed mutations at 2 different positions: 282 = TxC and 342 = CxT (NADH1_a) and the last one at 3 different positions: 27 = TxC; 181 = TxC and 282 = TxC (NADH1_b).
DISCUSSION
The present work is the first to compare and determine if there are differences in phenotypic and molecular features in E. granulosus cattle isolates from endemic areas in Argentina and Spain, where the abundance of this intermediate host as well as the importance in disease transmission are different.
In both regions, the only genotype infecting cattle was G1 (common sheep strain), which is the most important genotype responsible for human infection (7) and also because of its widespread distribution and wide range of intermediate hosts.
Although the G1, G2 and G5 genotypes have been found to infect cattle in Argentina (19, 21), all the isolates obtained in this study belonged to the G1 genotype. This suggests that G1 is probably the most prevalent and the principal genotype responsible for the hydatid disease in cattle and also in other hosts in the Argentinean area under study (3).
There are several studies where genetic differences within the G1 genotype of E. granulosus have been described (5, 6, 10, 19, 21, 32, 33). Obwaller et al. (2004) provided clear evidence that 'true' genetic variability exists within different genotypes indicating that this heterogeneity is not artefactual.
Results show the existence of different variants or microvariants of the G1 genotype in the Argentinean isolates but not in the Spanish ones. While the Spanish isolates presented the original sequences described for both genes (7, 9), in Argentina there are more than one variant for each gene. The variant for the CO1 gene with a transition mutation at position 73 found in the Argentinean samples had been registered earlier in this country as G1A (GenBank accession no. AF458871) in humans, dogs, cattle and pigs from different Argentinean provinces (19, 21), but also in cattle and sheep from Morocco (GenBank accession no. EF367289/90). The other variant for the CO1 with the transition mutation at position 111 found in this work had been previously registered as G1E variant (GenBank accession no. AF458875) in humans from Chile (21). This variant was also recorded in sheep from Piedmont, Italy (10), in dogs from China (GenBank accession no. DQ356882), in camels from Morocco (GenBank accession no. EF367286) and in cattle from Turkey (GenBank accession no. EU178103) and Australia (GenBank accession no. AJ508033). In the present work both variants were found in Argentinean cattle, being the first record of the G1E variant for the south-eastern region of Buenos Aires province. The ND1 gene variant (transition mutation at position 282) registered in this work has been previously found in sheep and humans in Argentina (33) (GenBank accession no. AF297617) but not in Spain, and this is the first record in cattle from the south-eastern region of Buenos Aires province for this variant. The other two ND1 variants were only found in one sample and not elsewhere using the BLAST search. Due to the low number of samples (one from each variant), no relationship could be drawn between these variants and biological factors. The variant with the mutation at position 282, which is the most prevalent in the Argentinean samples, was also described in Algeria (4) where the epidemiological situation reported is quite similar to that occurring in the endemic region of Argentina considered in this study. The variation intra G1 described here confirms that this is the most variable genotype. This is in agreement with the lowest intermediate host specificity and the wider geographic distribution detected for it. Also, our results confirm that the sequence of the ND1 gene fragment is more variable than that of the CO1 gene (9, 21, 29).
In most parts of the world G1 is mainly restricted to a sheep-dog cycle and cattle are usually considered as poor suitable hosts for this genotype (2, 39-41). This is the situation that exists in Central Spain, where sheep is the most important intermediate host for the disease. Spanish cattle were only infected with the G1 genotype and most of the isolates were non-viable and/or non-fertile (13, 26, 30, 36). However, in some countries like Tunisia and Algeria, the number of cattle with fertile cysts is higher. Therefore, this host has been considered a suitable host for the G1 genotype in those countries, suggesting that the parasite appears to be well adapted to the circumstances and is maintained in a cattle-dog cycle representing a source of infection to dogs and humans (4, 25). This situation is quite similar to the one that occurs in the southeastern region of Buenos Aires province, Argentina, where approximately 64% of the affected animals have fertile cysts: a similar percentage to those registered in Algeria (4). Also, the prevalence of the disease, the mean intensity of infection, the percentage of cysts by location and the variants or microvariants of the G1 genotype are almost the same as in Algeria but quite different from those in Spain indicating different epidemiological situations (4).
The differences registered between Argentina and Spain were remarkable, the mean intensity of infection was about three times higher in Argentina than in Spain and the mean intensity of viable cysts was more than 60 times higher in Argentina. Also, the total number of cattle considered in Argentina presented viable cysts whereas only 10% of the Spanish did so and the number of cattle with fertile cysts was 10 times higher in Argentina, indicating that cattle are suitable hosts and could play an important role in the transmission of the parasite in Argentina but not in Spain. However, while the mean intensity of liver infection was similar in Argentinean and Spanish cattle, the mean lung intensity was about four times higher in the Argentinean hosts, meaning that Argentinean cattle are infected with a higher number of lung cysts per host. Total mean intensity of infection and per organ (lung and liver) of viable and fertile cysts, the viable-to-totalcysts and the fertile-to-total cyst ratios were clearly higher in the Argentinean samples; however the total fertile-toviable- cysts ratio was higher in Spanish samples, although no differences were found in this ratio when lungs and liver cysts were analyzed separately. The low ratio of viable cysts in Spanish cattle indicates that the parasite does not develop in suitable conditions and most of the cysts die; however, the few viable ones can complete their development and therefore become fertile in the same proportion seen in viable cysts in the Argentinean cattle. This suggests that the main difference between both regions is the parasite's capability to infect and survive in cattle. Lungs are the most affected organs in both regions.
Regarding the predominance of cysts in lungs rather than in the liver, no agreement has been reached among different authors dealing with this subject (4, 17, 32, 39). According to García Llamazarez et al. (14), the greater number of cysts in lungs could be related to the fact that lungs show less organic reaction to external agents than liver. Nevertheless, there are several studies where the prevalence of hydatid cysts is greater in the liver, therefore, the location of cysts could depend on several factors such as the relationship between the oncosphere sizes and the lymphatic vessel sizes in the intermediate host (4, 17, 32, 39).
The ratios of viable-to-total cysts, fertile-to-total cysts and fertile-to-viable cysts are clearly higher in the liver. Another result, where the proportion of cattle fertile cysts was similar or greater in liver than in lungs, has been recorded in Benghazi, Libya (39). Therefore, viability and fertility are the most important phenotypic characteristics differentiating the Argentinean and Spanish G1 cattle isolates. The number of viable and fertile cysts registered in Spanish cattle were lower compared to those registered in Argentina. A possible explanation for this could be the result obtained from treating or not treating cattle with benzimidazole drugs, since this type of drugs interacts with metacestode viability and consequently, these parasites could present a higher level of survival which would reflect in a greater number of viable and fertile cysts (23). But the usage or not of these drugs in the Argentinean and Spanish cattle under study cannot be assured because this information was not collected for the aim of the present study.
Another methodology for strain determination is the use of morphological techniques, which is almost advantageous because it is a quick and cheap characterization method, especially in the field of epidemiology, whose results are compatible with genetic analyses (1, 2, 39). As regards the rostella, their arrangement and external aspect were similar in both regions and the values of the morphometric variables analyzed were within the range given by other authors for the common sheep strain (1, 2, 39, 40). However, there were significant differences in almost all the variables between the Argentinean and Spanish isolates. The results from the PCA analysis showing that all the morphological variables could be reduced to two functional ones coincide with previous studies where a multivariate analysis was performed (1, 3, 31). Also the PCA showed the existence of two distinct clearly separated groups: one corresponding to the Argentinean samples and the other to the Spanish ones.
In conclusion, the comparison of the epidemiological cysts features, of the larval rostellar hooks morphometry, and of the mitochondrial genes CO1 and ND1 sequences, showed some differences between cattle isolates from the endemic regions of Argentina and Spain studied in the present work. The most important phenotypic factor differentiating the Argentinean and Spanish cattle isolates is related to their viability and fertility features. In addition, the factorial analysis for morphometric data showed the existence of two distinct clearly separated groups (one corresponding to the Argentinean samples and the other to the Spanish ones). In addition, the comparison of sequence data showed differences within the G1 genotype. Since the G1 genotype microvariants for the mitochondrial genes CO1 and ND1 registered in the present study appear in cattle as well as in sheep from the same region of Argentina, we suggest that this genetic variability is independent from the host species or breed (3). Probably all the differences, from an epidemiological point of view, are related to the effectiveness of the control programmes of the hydatid disease in both countries. While in the southeastern region of Buenos Aires province the prevalence of the hydatid disease in cattle has not decreased between 1999 and 2004, in Spain the decrease in the prevalence of the disease in cattle, sheep and pigs between 2000 and 2005 was achieved through efficient control programmes (3, 11, 14, 15, 16).
Further studies involving other epidemiological, morphometric and molecular data from cattle and other intermediate hosts (like sheep, pigs, and horses) would contribute to expand this investigation.
Acknowledgements: The authors wish to acknowledge Mr. Oscar Chasma's help in the collection of the bovine material in Argentina. This work was partially supported by PICT-O ´02 Nº 08-11342, BID 1728/OC-AR and PIP Nº 02172, CONICET (Argentina).
1. Ahmadi NA. Using morphometry of the larval rostellar hooks to distinguish Iranian strains of Echinococcus granulosus. Ann Trop Med Parasitol 2004; 98: 211-20. [ Links ]
2. Ahmadi N, Dalimi A. Characterization of Echinococcus granulosus isolates from human, sheep and camel in Iran. Infect Genet Evol 2006; 6: 85-90. [ Links ]
3. Andresiuk MV. Determinación de cepas de Echinococcus granulosus y su importancia en la epidemiología de la hidatidosis-echinococcosis en el sudeste de la provincia de Buenos Aires. Tesis Doctoral, Universidad Nacional de Mar del Plata, Mar del Plata, Buenos Aires, Argentina, 2009. [ Links ]
4. Bardonnet K, Benchikh-Elfegoun MC, Bart JM, Harraga S, Hannache N, Haddad S et al. Cystic echinococcosis in Algeria: cattle act as reservoirs of a sheep strain and may contribute to human contamination. Vet Parasitol 2003; 116: 35-44. [ Links ]
5. Bart JM, Abdukader M, Zhang YL, Lin RY, Wang YH, Nakao M, et al. Genotyping of human cystic echinococcosis in Xinjiang, PR China. Parasitology 2006; 133: 571-9. [ Links ]
6. Bart JM, Morariu S, Knapp J, Ilie MS, Pitulescu M, Anghel A, et al. Genetic typing of Echinococcus granulosus in Romania. Parasitol Res 2006; 98: 130-7. [ Links ]
7. Bowles J, Blair D, McManus DP. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol Biochem Parasitol 1992; 54: 165-74. [ Links ]
8. Bowles J, Blair D, McManus DP. Molecular genetic characterization of the cervid strain (northern form) of Echinococcus granulosus. Parasitology 1994; 109: 215-21. [ Links ]
9. Bowles J, McManus DP. NADH dehydrogenase 1 gene sequences compared for species and strains of the genus Echinococcus. Int J Parasitol 1993; 23: 969-72. [ Links ]
10. Busi M, Snabel V, Varcasia A, Garippa G, Perrone V, De Liberato C, et al. Genetic variation within and between G1 and G3 genotypes of Echinococcus granulosus in Italy revealed by multilocus DNA sequencing. Vet Parasitol 2007; 150: 75-83. [ Links ]
11. Carmena D, Sánchez-Serrano LP, Barbero-Martýínez, I. Echinococcus granulosus Infection in Spain. Zoonoses Public Health 2008; 55: 156-65. [ Links ]
12. Cattell RB. The Scree Test for the number of factors. Multivariate Behav Res 1966; 1: 245-76. [ Links ]
13. Cuesta-Bandera C, McManus DP, Rishi AK. Characterization of Echinococcus granulosus of Spanish origin by DNA restriction endonuclease analysis and Southern blot hybridization. Int J Parasitol 1988; 18: 137-41. [ Links ]
14. Denegri GM, Elissondo M C, Dopchiz MC. Situación de la hidatidosis-echinococcosis en la República Argentina. Mar del Plata, Buenos Aires, Argentina, Editorial Martín, 2002. [ Links ]
15. Dopchiz MC. Aspectos epidemiológicos de la hidatidosisechinococcosis en el sudeste de la provincia de Buenos Aires. Mar del Plata, Buenos Aires, Argentina, Editorial Martin, 2006. [ Links ]
16. Dopchiz MC, Elissondo MC, Andresiuk MV, Maiorini E, Gutiérrez AM, Muzulin PM, et al. Pediatric hydatidosis in the south-east of the Buenos Aires province, Argentina. Rev Argent Microbiol 2009; 41: 105-11. [ Links ]
17. García Llamazares JL, Alvarez de Felipe AI, Redondo Cardeña PA, Prieto Fernández JG. Estudio de la fertilidad y viabilidad de quistes hidatídicos ovinos. Rev Esp Salud Pública 1997; 5: 445-9. [ Links ]
18. González LM, Daniel-Mwambete K, Montero E, Rosenzvit MC, McManus DP. Further molecular discrimination of Spanish strains of Echinococcus granulosus. Exp Parasitol 2002; 102: 46-56. [ Links ]
19. Haag KL, Ayala FJ, Kamenetzky L, Gutiérrez A, Rosenzvit M. Livestock trade history, geography, and parasite strains: the mitochondrial genetic structure of Echinococcus granulosus in Argentina. J Parasitol 2004; 90: 234-9. [ Links ]
20. Instituto Nacional de Estadísticas y Censos. INDEC. Sector Agropecuario. Ganadería. http://www.indec.mecon.ar. [ Links ]
21. Kamenetzky L, Gutiérrez AM, Canova SG, Haag KL, Guarnera EA, Parra A, et al. Several strains of Echinococcus granulosus infect livestock and humans in Argentina. Infect Genet Evol 2002; 2: 129-36. [ Links ]
22. Kedra HA, Swiderski Z, Tkach VV, Dubinski P, Paw³owski Z, Stefaniak J, et al. Genetic analysis of Echinococcus granulosus from humans and pigs in Poland, Slovakia and Ukraine. A multicentre study. Acta Parasitol 1999; 44: 248-54. [ Links ]
23. Lahmar S, Chéhida FB, Pétavy AF, Hammou A, Lahmar J, Ghannay A, et al. Ultrasonographic screening for cystic echinococcosis in sheep in Tunisia. Vet Parasitol 2007; 143: 42-9. [ Links ]
24. Lavikainen A, Lehtinen MJ, Meri T, Hirvela-Koski V, Meri S. Molecular genetic characterization of the Fennoscandian cervid strain, a new genotypic group (G10) of Echinococcus granulosus. Parasitology 2003; 127: 207-15. [ Links ]
25. Mrad S, Filisetti D, Oudni M, Mekki M, Belguith M, Nouri A, et al. Molecular evidence of ovine (G1) and camel (G6) strains of Echinococcus granulosus in Tunisia and putative role of cattle in human contamination. Vet Parasitol 2005; 129: 267-72. [ Links ]
26. Mwambete DK, Ponce-Gordo F, Cuesta-Bandera C. Genetic identification and host range of the Spanish strains of Echinococcus granulosus. Acta Trop 2004; 91:87-93. [ Links ]
27. Nakao M, McManus DP, Schantz PM, Craig PS, Ito A. A molecular phylogeny of the genus Echinococcus inferred from complete mitochondrial genomes. Parasitology 2007; 134: 713-22. [ Links ]
28. National Center for Biotechnology Information. NCBI. http://www.ncbi.nlm.nih.gov. [ Links ]
29. Obwaller A, Schneider R, Walochnik J, Gollackner B, Deutz A, Janitschke K, et al. Echinococcus granulosus strain differentiation based on sequence heterogeneity in mitochondrial genes of cytochrome c oxidase-1 and NADH dehydrogenase-1. Parasitology 2004; 128: 569-75. [ Links ]
30. Ponce Gordo F. Caracterización biológica de los genotipos de Echinococcus granulosus de origen español. Tesis Doctoral, Universidad Complutense de Madrid, Madrid, España, 1995. [ Links ]
31. Ponce Gordo F, Cuesta Bandera C. Differentiation of Spanish strains of Echinococcus granulosus using larval rostellar hook morphometry. Int J Parasitol 1997; 27: 41-9. [ Links ]
32. Rinaldi L, Maurelli MP, Capuano F, Perugini AG, Veneziano V, Cringoli S. Molecular update on cystic echinococcosis in cattle and water buffaloes of southern Italy. Zoonoses Public Health 2008; 55: 119-23. [ Links ]
33. Rosenzvit MC, Zhang LH, Kamenetzky L, Canova S, Guarnera EA, McManus DP. Genetic variation and epidemiology of Echinococcus granulosus in Argentina. Parasitology 1999; 118: 523-30. [ Links ]
34. Saarma U, Jõgisalu I, Moks E, Varcasia A, Lavikainen A, Oksanen A, et al. A novel phylogeny for the genus Echinococcus based on nuclear data challenges relationships based on mitochondrial evidence. Parasitology 2009; 21: 1-12. [ Links ]
35. Scott JC, Stefaniak J, Pawlowski ZS, McManus DP. Molecular genetic analysis of human cystic hydatid cases from Poland: identification of a new genotypic group (G9) of Echinococcus granulosus. Parasitology 1997; 114: 37-43. [ Links ]
36. Siles-Lucas M, Benito MC, Cuesta-Bandera C. Echinococcus granulosus: genomic and isoenzymatic study of Spanish strains isolated from different intermediate hosts. Vet Parasitol 1996; 63: 273-82. [ Links ]
37. Siles-Lucas M, Felleisen R, Cuesta-Bandera C, Gottstein B, Eckert J. Comparative genetic analysis of Swiss and Spanish isolates of Echinococcus granulosus by southern hybridization and Random Amplified Polymorphic DNA technique. Appl Parasitol 1994; 35: 107-17. [ Links ]
38. Smyth JD, Davies Z. Occurrence of physiological strains of Echinococcus granulosus demonstrated by in vitro culture of protoscoleces from sheep and horse hydatid cysts. Int J Parasitol 1974; 4: 443-5. [ Links ]
39. Tashani OA, Zhang LH, Boufana B, Jegi A, McManus DP. Epidemiology and strain characteristics of Echinococcus granulosus in the Benghazi area of eastern Libya. Ann Trop Med Parasitol 2002; 96: 369-81. [ Links ]
40. Thompson RCA, Kumaratilake LM, Eckert J. Observations on Echinococcus granulosus of cattle origin in Switzerland. Int J Parasitol 1984; 14: 283-91. [ Links ]
41. Thompson RCA, McManus DP. Towards a taxonomic revision of the genus Echinococcus. Trends Parasitol 2002; 18: 452-7. [ Links ]
Recibido: 11/08/09
Aceptado: 29/10/09














