Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Revista argentina de microbiología
versão impressa ISSN 0325-7541versão On-line ISSN 1851-7617
Rev. argent. microbiol. v.42 n.1 Ciudad Autónoma de Buenos Aires jan./abr. 2010
ARTÍCULO ORIGINAL
Preparation of a working seed lot of BCG and quality control by PCR genotyping
A. Trovero1*, C. Argüelles1, A. Cataldi2
1Instituto Nacional de Producción de Biológicos (INPB), ANLIS "Dr. Carlos G. Malbrán". Av. Vélez Sarsfield 563, (1281) Ciudad Autónoma de Buenos Aires;
2Instituto de Biotecnología, CNIA-INTA Castelar, Prov. de Buenos Aires, Argentina.
*Correspondence. E-mail: atrovero@anlis.gov.ar
ABSTRACT
The bacillus Calmette-Guérin (BCG) was obtained in 1920 after successive passages leading to the attenuation of a Mycobacterium bovis strain. For the following 40 years, BCG had been replicated, resulting in substrains with genotypic and phenotypic differences. Several genomic studies have compared two BCG strains, M. bovis and Mycobacterium tuberculosis, and observed that deleted regions in the different strains could be related to differences in antigenic properties. In this work, a working seed lot was obtained from a lyophilized secondary seed lot from the BCG Pasteur strain 1173 P2 and genetically characterized. The genome was analyzed by PCR directed to five regions (RD1, RD2, RD14, RD15, DU2), using the seed lot and different available strains as templates. No genetic differences were found in the fragments studied as compared to the Pasteur strain. A total of 20 passages were carried out and no differences were found in the size of the fragments amplified by PCR. In conclusion, this method allows to control a working seed lot genotypically and to assess the stability of the BCG genome.
Key words: BCG strains; Genome; Working seed lot; PCR; Quality control
RESUMEN
Preparación de un lote semilla de trabajo de BCG y control de calidad por genotipificación por PCR. El bacilo de Calmette-Guérin (BCG) se obtuvo en 1920, después de sucesivos pasajes que llevaron a la atenuación de una cepa de Mycobacterium bovis. A lo largo de los 40 años subsiguientes la cepa BCG fue replicada y surgieron subcepas con diferencias fenotípicas y genotípicas. Se realizaron varios estudios de comparación genómica de diferentes cepas de BCG, M. bovis y Mycobacterium tuberculosis, y se observó que las deleciones de regiones en las diferentes cepas podrían estar relacionadas con diferencias en las propiedades antigénicas. En este trabajo se describe la preparación y caracterización genética de un lote semilla de trabajo obtenido a partir de un lote semilla secundaria liofilizado de la cepa BCG Pasteur 1173 P2. Se analizaron por PCR cinco regiones (RD1, RD2, RD14, RD15, DU2) en el lote semilla de trabajo utilizando como control las diferentes cepas disponibles. No se hallaron diferencias genéticas en los fragmentos estudiados al comparar el lote semilla de trabajo con la cepa BCG Pasteur 1173 P2. Asimismo, se efectuaron hasta 20 pasajes y no se encontraron diferencias en el tamaño de los fragmentos amplificados por PCR. En conclusión, se ha puesto a punto un método que permite controlar el genotipo de un lote semilla de trabajo y evaluar la estabilidad del genoma del BCG.
Palabras clave: Cepas BCG; Genoma; Lote semilla de trabajo; PCR; Control de calidad
INTRODUCTION
The history of the bacillus Calmette-Guérin (BCG) started in 1908 when Calmette and Guérin began their work from a virulent strain of Mycobacterium bovis by performing serial passages in a bile-containing medium. For the following thirteen years, a total of 230 passages were performed until the M. bovis strain lost its virulence in animals. The original BCG strain had then been maintained and distributed worldwide, since 1921 for over 40 years (24). It was noted that several BCG strains maintained by continuous subcultures suffered phenotypic changes (14, 28). In 1950, the World Health Organization (WHO) gave the first recommendation for the production and control of BCG vaccines. Since 1960, the Pasteur strain has been freeze-dried, keeping the form of a primary seed lot. There are heterogeneous phenotypic characteristics in vitro and in vivo among the different strains. Behr et al. (5) collected data on the distribution of different strains. According to historical records, the first distribution of a documented daughter strain is the BCG Russia obtained in 1924. From then on, until its lyophilization in 1961, BCG-Pasteur daughter strains were obtained after 1173 passages, either directly or indirectly at irregular times. After BCG Russia, the following strains were obtained: Moreau (1925), Japan (1925), Sweden (1926) and Park (1926 given to Phipps 1928) and Denmark (1931), Tice (1934), Frappier (1937), Birkhaug (1946), Connaught (1948 from Frappier), Prague (1947 from Denmark), Glaxo (1954 from Denmark) and Pasteur (lyophilized in 1961 after 1173 serial passages) (5, 24).
Much later, the genome sequence of M. tuberculosis (H37Rv and CDC1551) (13, 16) and the sequence of two M. bovis strains (BCG Pasteur and AF2122/97) became available (17). Since then, these strains have been used as references in comparative genomic studies. The comparison of genomes between different strains and/or species of mycobacteria has led to the identification of genomic differences that may explain the observed phenotypic differences, such as host range, virulence and pathogenesis. In addition, the analysis of deletions by differential hybridization has allowed the construction of a phylogenetic tree of the species of the M. tuberculosis complex (10). The different BCG strains have been studied by comparative genomics, subtractive genomic hybridization (11, 18, 22), or DNA microarray technologies (6), generating deletions and insertions known as regions of difference (RD). Although the BCG genome has undergone a number of deletions, it is 30 kb longer than the wild type M. bovis AF2122/97, resulting from two tandem duplications, DU1 and DU2. The comparison of the genome sequences of M. tuberculosis with those of M. bovis and BCG Pasteur led to find 42 RD. These comprise 170 genes, of which BCG Pasteur has lost 133 (9).
Although new tuberculosis vaccines are currently under investigation (1), BCG is the cornerstone of the strategy to fight tuberculosis. The genetic differences between BCG vaccine strains have renewed the interest in the influence of the vaccine strain on the protective efficacy against tuberculosis. Although there is good evidence to support the notion that the induced immune response and protection afforded against tuberculosis differs between BCG vaccine strains, there are currently insufficient data to favour or recommend one particular strain (2, 4, 7, 8, 12, 20, 23, 26).
In our laboratory, the production of an intravesical BCG for cancer has been attained from a lyophilized secondary seed lot Pasteur strain. The seed is cultivated in synthetic liquid culture media (Sauton medium). The production is performed according to the recommendations of the WHO, with the "Seed Lot System". To avoid the occurrence of mutants and possible changes in immunogenicity, it is recommended that no more than 12 passages should be performed in culture medium from the primary seed lot. According to the WHO´s recommendations, the controls that should be performed are the microscopic observation of bacilli, Ziehl-Neelsen staining, the determination of the aspect of the colonies, the exclusion of bacterial or fungal contamination, and the absence of virulent mycobacteria (30).
The aim of this study was to obtain a working seed lot of BCG from a Pasteur 1173P2 secondary seed lot and to perform a molecular characterization of the batch obtained, since it may serve as a genetic quality control for future lots.
MATERIAL AND METHODS
Production of a working seed lot
The working seed lot was obtained by conventional cultivation as a film surface in Sauton media, from strain BCG1173P2, with only two passages, according to the recommendation of the WHO (27, 30). The cultures were separated from their media by filtration in a Birkhaug funnel and rinsed with sterile sodium glutamate 1.5% w/v. A total of 20 g of bacteria was transferred to another flask with stainless steel balls to disperse the bacilli by rotation of the flask for 25 minutes at 40 RPM. The bacillary mass was then resuspended in 1.5% sodium glutamate at a concentration of 20 mg/ml. These preparations were lyophilized and frozen at -25 °C at vacuum. After 12 h, the secondary drying started at a temperature of 18 °C, in a vacuum chamber and stored at 4 °C.
Strains
Connaught (Pasteur Merieux Connaught-Canada), Danish (Staten Serum Institut-Copenhague), Pasteur 1173 P2 (Pasteur Institute), Russia (BB-NCPID Ltd. Bulgaria), Glaxo (Glaxo Laboratories), and M. bovis AN5.
Purification of genomic DNA
DNA was purified from BCG colonies on solid Lowenstein- Jensen according to the method described by van Soolingen et al. (29).
PCR amplification
PCR was performed on fragments related to regions RD1, RD2, RD14, RD15, duplications DU2-I, DU2-III and DU2-IV, and the internal deletion of DU2, to distinguish different strains of BCG. Primers (See Table 1) were used for the internal and flanking regions of RD2 and RD14. For duplications, internal primers with opposite direction were used. The nomenclature of RD and duplicated regions was used as by Brosch et al. (9). For preliminary genome comparisons between M. tuberculosis and M. bovis BCG, web sites http://genolist.pasteur.fr/TubercuList/ and http://genolist.pasteur.fr/BCGList were used. Primers for RD 15 were designed using the Primer3 web site http://wwwgenome.wi.mit.edu/cgi-bin/primer/primer3. A PCR was performed with the different BCG strains and the working lot, and with successive passages in Sauton liquid medium. The PCR reaction was performed in a final volume of 50 μl with 1.25 U of Promega Taq polymerase, 2 mM MgCl2, 0.05 mM dNTP and 50 nM of each primer. The PCR conditions were 1 cycle of 96 °C (4 min), 29 cycles of 96 °C (1 min), between 50-60 °C [depending on the annealing temperature (Ta) for each pair of primers, Table 1] for 1 min, 72 °C (2 min) and 1 cycle of 72 °C (10 min). The reaction was carried out in a Perkin Elmer thermocycler. The PCR products were analyzed by electrophoresis in horizontal 1.2% agarose gels, with ethidium bromide.
RESULTS
Controls were performed according to the WHO standards: in the seed lot and other control BCG strains, acidfast bacilli in Ziehl-Neelsen staining with small clumps, which are typical of BCG, were observed. In Lowenstein- Jensen, the bacilli presented themselves as expanded colonies without pigment and with slow growth. In the test of purity in trypticase soy broth and thioglycolate broth, there was no development of bacteria or fungi.
There was a survival of 60% of guinea pigs after inoculation with the BCG seed lot. The average initial weight of the surviving guinea pigs was 398 g and the average final weight 763 g. The average weight gain in six months was 365 g. The guinea pigs were killed after six months and no signs of tuberculosis were found. According to the WHO recommendations the result was satisfactory.
Molecular characterization
RD1 region: all BCG strains studied gave an amplification product of approximately 500 bp, with primers flanking the RD1 region, indicating a deletion of RD1, whereas the DNA of M. bovis wild type was not amplified (Figure 1).

Figure 1. PCR corresponding to the RD1 region. Lanes: 1- DNA ladder, 2- BCG Connaugt, 3- BCG Danish, 4- BCG Pasteur, 5- BCG Glaxo, 6- BCG Russia, 7- Working seed lot, 8- M. bovis AN5.
RD2 region: only the Russia strain produced an amplification product of approximately 500 bp with primers internal to the region, while the other strains did not show such product, because they have a deletion of that region. With primers flanking the region, a band of approximately 1 kb confirmed the existence of a deletion of approximately 10 kb region of RD2 in all the strains, except for the Russia strain, where the 1 kb band was not observed (Figure 2).

Figure 2. Left: PCR corresponding to the RD2 internal region. Right: PCR corresponding to the RD2 flanking region. Lanes: 1- BCG Connaught, 2- BCG Danish, 3- BCG Pasteur, 4- BCG Glaxo, 5- BCG Russia, 6- Working seed lot, 7- negative control, 8- DNA ladder.
RD14 region: the Pasteur strain and the working lot gave a 500 bp fragment in the amplification with primers flanking RD14, but gave no amplification with the internal primers. Therefore, these strains have a deletion of the RD14 region. The other strains gave a 470 bp fragment with the internal primers, and no amplification with the external ones. As previously described, these strains have the RD14 region (Figure 3).

Figure 3. Left: PCR corresponding to the RD14 internal region. Lanes: 1- BCG Connaught, 2- BCG Danish, 3- BCG Glaxo, 4- BCG Pasteur, 5- BCG Russia, 6-Working seed lot, 7- negative control, 8- DNA ladder. Right: PCR corresponding to the RD14 flanking region: Lanes: 1- BCG Connaught, 2- BCG Danish, 3- BCG Glaxo, 4- BCG Russia, 5- BCG Pasteur, 6- Working seed lot, 7- negative control, 8- DNA ladder.
RD15 region: this region is present in all BCG strains tested, except for the Connaught strain. These data are consistent with previous results (5, 6, 9), since there was an amplification product of approximately 500 bp with primers internal to the region in all the strains except for Connaught (Figure 4).
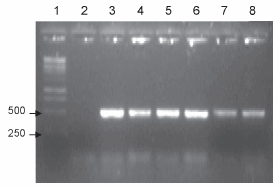
Figure 4. PCR corresponding to the RD15 region. Lanes: 1- BCG Connaught, 2- BCG Danish, 3- BCG Pasteur, 4- BCG Glaxo, 5- BCG Russia, 6- Working seed lot, 7- DNA ladder; 8- negative control.
DU2 group I duplication: the amplification with the Russia strain gave a 500 bp fragment. This coincides with the existence of the duplication described by Brosch et al. (9) in that strain. In the other strains, no band was observed, thus indicating that there is no such duplication in these strains, including the working lot (Figure 5).
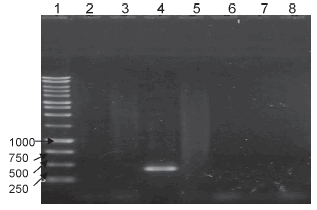
Figure 5. PCR corresponding to the DU2-group 1 duplication. Lanes: 1- DNA ladder, 2- BCG Connaught, 3- BCG Danish, 4- BCG Russia, 5- BCG Glaxo, 6- BCG Pasteur 1173P2, 7- Working seed lot, 8- negative control.
DU2 group III duplication: this amplification was observed with the Danish and Glaxo strains, yielding a fragment slightly smaller than 500 bp which corresponds to the duplication described by Brosch et al. (9). There was no amplification band in the working lot, the Pasteur, the Connaught and the Russia strains, thus concluding that there is no such duplication in these strains (Figure 6).

Figure 6. PCR corresponding to the DU2-Group III duplication. Lanes: 1- DNA ladder, 2- BCG Connaught, 3- BCG Danish, 4- BCG Russia, 5- BCG Glaxo, 6- BCG Pasteur 1173P2, 7- Working seed lot.
DU2 group IV duplication: in the amplification with the Connaught and Pasteur strains, there is a 500 bp fragment that corresponds to the duplication of the DU2 group IV described by Brosch et al. (9). In the working seed lot and the Pasteur strain, the band was observed, thus concluding that the DU2 duplication is present in these strains (Figure 7).

Figure 7. PCR corresponding to the DU2-Group IV duplication. Lanes: 1- BCG Connaught, 2- BCG Danish, 3- BCG Russia, 4- BCG Glaxo, 5- BCG Pasteur 1173P2, 6- Working seed lot, 7- negative control, 8- DNA ladder.
Internal deletion of DU2 duplication: with the primers that flank the deletion, a fragment shorter than 500 bp was amplified in all strains except in the Russia strain, thus suggesting that in this region there is a deletion of about 60 kb, as described by Brosch et al. (9) (Figure 8).

Figure 8. PCR corresponding to internal deletion of DU2. Lanes: 1- DNA ladder, 2- BCG Connaught, 3- BCG Danish, 4- BCG Russia, 5- BCG Glaxo, 6- BCG Pasteur 1173P2, 7- Working seed lot, 8- negative control.
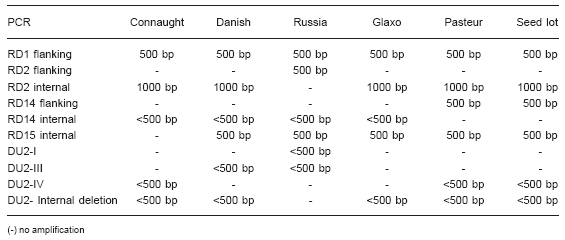
The results are summarized in Table 2.
Table 2. Results of the PCR amplification with the different BCG strains
Genomic stability after serial passages: PCR reactions were tested with the working seed lot alter 3, 6, 10, 15 and 20 passages in Sauton medium and no differences were observed when compared to the initial working seed lot culture.
Phenotypic characterization: enzymatic biochemical characterization was performed in the Pasteur BCG strain and the seed lot, and both gave similar results. Catalase at 25 °C and urease were positive, and the following tests were negative: catalase at 68 °C, nitrate reductase, hydrolysis of Tween 80, b-glucosidase, b-galactosidase, pyrazinamidase, arylsulphatase, and iron reduction. Susceptibility tests to antibiotics were also performed to the Pasteur BCG and the seed lot strains by the proportion method on Löwenstein Jensen medium. Both gave similar results, they were susceptible to: hydrazide of phenocarboxylic acid 2 μg/ml, aminosalicylic acid 0.5 μg/ ml, isoniazid 0.2 μg/ml, streptomycin 4 μg/ml, ethambutol 2 μg/ml, and rifampicin 40 μg/ml; they were resistant to cycloserine 30 μg/ml.
DISCUSSION
In this work, we produced and genetically characterized a working seed lot of BCG, which may be used in the production of an intravesical BCG or intradermal vaccine. This methodology allows to standardize the production process, with a limited number of passages. We established a procedure with only two passages from the seed working lot, and therefore, only four from the Pasteur 1173P2 secondary seed lot, used to start the seed lot.
The importance of reducing the number of passages is that upon subculturing, the bacteria can undergo genetic changes and, depending on the culture conditions, mutants may be selected. In fact, this happened with the original BCG strain that originated the different substrains that are available at present. Although the WHO recommends to limit the number of passages to 12 (30), it is preferable to reduce them to 3 (31-33). In the laboratory production of BCG during subcultures, changes, such as deletions or duplications, may occur. These mutations can alter the strain phenotypically and may be involved in the virulence, survival, and induction of immune responses. The attenuation is generated by deletions of virulence genes (19, 21, 25). Reversing the mutation is impossible, but mutations would further attenuate the strain and generate a decrease in protective efficacy. In order to avoid the occurrence of mutants during the production process and to reduce the number of passages, we produced a seed working lot, which will be involved in the production process with only two passages.
On the other hand, the seed working lot was genetically characterized, to assess genomic stability. We analyzed regions that characterize the different strains available by PCR. Five regions (RD1, RD2, RD14, RD15, DU2) were analyzed and primers directed outward from the DU2 duplicated region were used to find out whether or not there was a duplication.
No changes were found in the working seed lot as compared to the Pasteur strain in the regions studied or after twenty successive passages.
We herein describe a PCR technique that is simple and economical, and that can be performed in any quality control laboratory of biologics. A more thorough analysis of the genome can be carried out with other molecular biology techniques, such as microarray hybridization or pulsed field electrophoresis (34). A multiplex PCR method that analyzed six regions, but did not include the tandem duplications, has been previously described (3). The methodology presented here uses internal and flanking primers, and allows to detect the presence of mixed cultures with and without deletions.
Another advantage of PCR in this work is the discrimination of BCG strains. If we take into account that different strains are available in the country for the BCG vaccine and bladder cancer treatment, it is important to have a potential method for the identification and characterization of BCG. In the case of any complication such as sepsis or infection in vaccinated patients or in patients with bladder cancer treatment, the causative organism can be identified because the method can distinguish different BCG strains as well as M. bovis from M. tuberculosis.
In summary, a working seed lot was prepared and genetically characterized, following the controls recommended by the WHO (30) and the Argentine Pharmacopoeia (15), with satisfactory results.
1. Andersen P, Doherty M. The success and failure of BCG implications for a novel tuberculosis vaccine. Nat Rev Microbiol 2005; 3: 656-61. [ Links ]
2. Baily GV. Tuberculosis prevention trial, Madras. Indian J Med Res 1980; 72: 1-74. [ Links ]
3. Bedwell J, Kairo S, Behr M, Bygraves J. Identification of substrains of BCG vaccine using multiplex PCR. Vaccine 2001; 19: 2146-51. [ Links ]
4. Behr MA. Correlation between BCG genomics and protective efficacy. Scand J Infec Dis 2001; 33: 249-52. [ Links ]
5. Behr MA, Small PM. A historical and molecular phylogeny of BCG strains. Vaccine 1999; 17: 915-22. [ Links ]
6. Behr MA, Wilson M, Gill W, Salamon H, Schoolnik G, Rane S, et al. Comparative genomics of BCG vaccines by wholegenome DNA microarray. Science 1999; 284: 1520-3. [ Links ]
7. Bonifachich E, Chort M, Astigarraga A, Diaz N, Brunet B, Bottasso E. Protective effect of bacillus Calmette-Guérin (BCG) vaccination in children with extra-pulmonary to pulmonary disease A case-control study in Rosario, Argentina. Vaccine 2006; 24: 2894-9. [ Links ]
8. Brewer T. Preventing tuberculosis with bacillus Calmette- Guérin vaccine: a meta-analysis of literature. Clin Infect Dis 2000; 31: S64-7. [ Links ]
9. Brosch R, Gordon S, Garnier T, Eiglmeier K, Frigui W, Valenti P, et al. Genome plasticity of BCG and impact on vaccine efficacy. Proc Natl Acad Sci USA 2007; 104: 5596-601. [ Links ]
10. Brosch R, Gordon S, Marmiesse M, Brodin P, Buchrieser C, Eiglmeier K, et al. A new evolutionary scenario for Mycobacterium tuberculosis complex. Proc Natl Acad Sci USA 2002; 99: 3684-9. [ Links ]
11. Brosch R, Gordon S, Pym A, Eiglmeier K, Garnier T, Cole S. Comparative genomics of mycobacteria. Int J Med Microbiol 2000; 290: 143-52. [ Links ]
12. Colditz G, Brewer T, Berkey C, Wilson ME, Burdick E, Fineberg HV, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. JAMA 1994; 271: 698-702. [ Links ]
13. Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 1998; 393: 537-43. [ Links ]
14. Dubos RJ, Pierce CH. Differential characteristics in vitro and in vivo of several substrains of BCG. Am Rev Tuberc Scand 1948; 22: 125-33. [ Links ]
15. Farmacopea Argentina. ANMAT, séptima edición. Argentina, 2002. [ Links ]
16. Fleischmann RD, Alland D, Eisen JA, Carpenter L, White O, Peterson J, et al. Whole-genome comparison of Mycobacterium tuberculosis clinical and laboratory strains. J Bacteriol 2002; 184: 5479-90. [ Links ]
17. Garnier T, Eiglmeier K, Camus J, Medina N, Mansoor H, Pryor M, et al. The complete genome sequence of Mycobacterium bovis. Proc Natl Acad Sci USA 2003; 100: 7877-82. [ Links ]
18. Gordon S, Brosch R, Billault A, Garnier T, Eiglmeier K, Cole S. Identification of variable regions in the genomes of tubercle bacilli using bacterial artificial chromosome arrays. Mol Microbiol 1999; 32: 643-55. [ Links ]
19. Hsu T, Hingley-Wilson S, Chen B, Chen M, Dai A, Morin P, et al. The primary mechanism of attenuation of bacillus Calmette-Guérin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc Natl Acad Sci USA 2003; 100: 12420-5. [ Links ]
20. Lagranderie M, Balazuc A, Deriaud E, Leclerc C, Gheorghiui M. Comparison of immune responses of mice immunized with five different Mycobacterium bovis BCG vaccine strains. Infect Immun 1996; 64: 1-9. [ Links ]
21. Lewis K, Liao R, Guinn K, Hickey M, Smith S, Behr M, Sherman D. Deletion of RD1 from Mycobacterium tuberculosis mimics bacile Calmette-Guérin attenuation. J Infect Dis 2003; 187: 117-23. [ Links ]
22. Mahairas G, Sabo P, Hickey M, Singh D, Stover K. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol 1996; 178: 1274-82. [ Links ]
23. Miceli I, Kantor I, Colaiacovo D, Peluffo G, Cutillo I, Gorra R, et al. Eficacia de la vacunación con BCG evaluada mediante el método de casos y testigos en Buenos Aires, Argentina. Bol Oficina Sanit Panam 1988; 104: 440-8. [ Links ]
24. Mostowy S, Tsolaki A, Small P, Behr M. The in vitro evolution of BCG. Vaccine 2003; 21: 4270-4. [ Links ]
25. Pym A, Brodin P, Brosch R, Huerre M, Cole S. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol Microbiol 2002; 46: 709-17. [ Links ]
26. Ritz N, Hanekom W, Robins-Browne R, Britton W, Curtis N. Influence of BCG vaccine strain on the immune response and protection against tuberculosis. FEMS Microbiol Rev 2008; 32: 821-41. [ Links ]
27. Sáenz A. Método de producción de vacuna BCG líquida de uso intradérmico Rev Argent Tuberc Enferm Pulm 1971; 22: 106-24. [ Links ]
28. Suter W, Dubos R. Variability of BCG strains (bacillus Calmette Guérin) J Exp Med 1951; 93: 559-72. [ Links ]
29. Van Soolingen D, Hermans P, Haas P, Soll D, Van Embden J. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J Clin Microbiol 1991; 29: 2578-86. [ Links ]
30. WHO Technical Report Series, No 745, 1987. Requirements for dried BCG vaccine. Geneva, Switzerland. [ Links ]
31. WHO Meeting Report. Consultation on the characterization of BCG strains. London, England, 15-16 December 2003. [ Links ]
32. WHO Report. Consultation on the characterization of BCG vaccines. Geneva, Switzerland, 8-9 December 2004. [ Links ]
33. WHO Report. Discussion on the improvement of quality control of BCG vaccines. Pasteur Institute, Paris, France, 7 June 2005. [ Links ]
34. Zhang Y, Wallace R, Mazurek G. Genetic differences between BCG substrains. Tuber Lung Dis 1995; 76: 43-50. [ Links ]
Recibido: 21/07/09
Aceptado: 24/11/09















