Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista argentina de microbiología
Print version ISSN 0325-7541On-line version ISSN 1851-7617
Rev. argent. microbiol. vol.42 no.1 Ciudad Autónoma de Buenos Aires Jan./Apr. 2010
INFORME BREVE
The effect of Mycoplasma and mycoplasma removal agent on the hydrolase activity in fibroblasts of patients with lysosomal diseases
F. T. S. Souza1, 2*, L. S. Sostruznik1, 2, R. C. Scolari1, 2 K. J. M. Castro1, 2, C. V. Andrade 2, R. Giugliani2, 3, J. C. Coelho1, 2
1Departamento de Bioquímica, ICBS, Universidade Federal do Rio Grande do Sul;
2Serviço de Genética Médica, Hospital de Clínicas de Porto Alegre;
3Departamento de Genética, IB, Universidade Federal do Rio Grande do Sul. Porto Alegre, RS, Brazil.
*Correspondence. E-mail:fernandatimm@hotmail.com
ABSTRACT
This study was designed to evaluate the effect of mycoplasma contamination on acid hydrolase activity and the action of the mycoplasma removal agent (MRA), in cultures of human fibroblasts from individuals with lysosomal diseases. For this purpose, we measured the activity of the b-galactosidase, arylsulphatase B (ASB), hexosaminidase A and a-glucosidase enzymes. The activity of the above mentioned enzymes in fibroblasts contaminated by mycoplasma was measured before and after the addition of the MRA. The results were then compared to the enzymatic activity in contamination-free cultures. Only the ASB enzyme showed significant alteration in activity both in the presence of mycoplasma and MRA. The remaining enzymes did not suffer significant interference by the presence of the two agents. Of the four enzymes tested, three did not suffer significant alterations by the presence of the mycoplasma nor from the MRA. However, the activity measured in the ASB enzyme increased significantly in the presence of mycoplasma and MRA and could lead to a doubtful diagnosis. Therefore, we suggest that contamination should be prevented by using aseptic techniques as well as the MRA in those fibroblast cultures that cannot be discarded.
Key words: Mycoplasma; Fibroblast culture; Lysosomal hydrolases; Lysosomal diseases
RESUMEN
Efecto de Mycoplasma y del agente de eliminación de micoplasmas en la actividad de las hidrolasas en fibroblastos de pacientes con enfermedades lisosomales. Este estudio fue diseñado para evaluar el efecto de la contaminación por micoplasmas sobre la actividad de hidrolasas ácidas y la acción del agente de eliminación de micoplasmas (MRA) en cultivos de fibroblastos humanos de pacientes con enfermedades lisosomales. Se midió la actividad de la b-galactosidasa, arilsulfatasa B (ASB), hexosaminidasa A y a-glucosidasa en estos cultivos. La actividad de estas enzimas en los fibroblastos contaminados por micoplasmas se midió antes y después de la adición de MRA. Los resultados se compararon con los obtenidos en cultivos libres de contaminación. Sólo la enzima ASB demostró alteración significativa en la actividad, tanto en presencia de micoplasmas como con la adición de MRA. Las enzimas restantes no sufrieron alteraciones significativas en presencia de micoplasmas, ni tras la adición de MRA. La actividad medida para la enzima ASB aumentó significativamente en presencia de micoplasmas y MRA, lo que podría conducir a un diagnóstico dudoso. Por lo tanto, sugerimos evitar la contaminación con micoplasmas mediante el uso de técnicas asépticas y la utilización de MRA en los cultivos de fibroblastos que no se puedan descartar.
Palabras clave: Mycoplasma; Cultivo de fibroblastos; Hidrolasas lisosomales; Enfermedades lisosomales
Mycoplasma contamination has been known since the beginning of cell culture technology (7) and is a recurring problem in cell culture laboratories, especially those that work with human cells. Reports from various countries show that 10 to 87% of cell cultures are infected by mycoplasma (6). The degree of contamination depends directly on the contaminating species, the control practices utilized, the efficiency of the procedures and the type of test applied. Treatment with antibiotics is still considered the most effective procedure for elimination or deactivation of mycoplasma in cell cultures. However, antimicrobial therapy is not successful in timely decontamination and undesirable cytotoxicity effects can result in cell death as well as in developing bacteria resistant to the antibiotic (15).
In a study with fibroblast cultures from normal individuals, we found that the activity of some lysosomal hydrolases increases significantly in the presence of mycoplasma. When we used the 4-oxo-quinolone-3-carboxylic acid antibacterial agent known as mycoplasma removal agent (MRA) in these cultures, the mycoplasma was eliminated and the enzymatic activity remained unaltered. On the other hand, when we used MRA in non contaminated cultures, it could be noted that this agent increased the enzymatic activity of the hydrolases analysed. It suggests that, in the same way as the mycoplasma, this quinolonetype antibacterial agent could also be an interference factor in the measurement of enzymatic activity (13).
In the present study we investigate the behavior of four lysosomal hydrolases (b-galactosidase, arylsulphatase B, hexosaminidase A, and a-glucosidase) in primary fibroblasts cultures obtained by a forearm skin biopsy from patients with lysosomal disorders (LD). The cultures were contaminated with mycoplasma and subsequently treated with the MRA. The aim of this work was to investigate the interference of mycoplasma and/or MRA on the enzymatic hydrolase activity.
The LDs used in this study were: GM1 gangliosidosis (b-galactosidase deficiency), mucopolysaccharidosis type VI (arylsulphatase B deficiency), Tay-Sachs disease (hexosaminidase A deficiency) and Pompe´s disease (a-glucosidase deficiency). These diseases by themselves can cause a generalized disorganization of the cellular components (1) by the accumulation of non-degradable substrates.
The cultures were divided into three groups: The control group (cont 0): mycoplasma-free cultures, n = 5 (Figure 1A); the test group 0 (test 0): culture infected spontaneously by undefined species of mycoplasma, n = 5 (Figure 1B); the test group 1 (test 1): contaminated cultures treated with MRA, n = 5.

Figure 1. Mycoplasma detection by Hoescht Stain Solution. (A) Control group: Mycoplasma-free cultures; (B) Test Group: Cell cultures with mycoplasma
Cells were cultured in tissue culture flasks (25 cm2; Corning Incorporated Corning, N.Y.) containing 3 ml of nutrient HAM-F10 (Cultilab Mat. Cult. Cel. Ltda, Campinas, SP, Brazil), supplemented with 20% fetal calf serum (FCS) (GIBCO laboratories, Grand Island, N.Y.). The cultures were kept in an incubator at 37 °C (4). All tasks were performed in class II biosafety cabinets. Mycoplasma was detected by the use of a mycoplasma Stain Kit from Sigma. In this kit, fibroblasts were stained with a "Hoechst Stain" solution (3). Stained cells were observed with fluorescence microscopy with G365 excitation filters, an LP 420 barrier filter and an FT 395 chromatic beam splitter. These cells were kept in culture until we obtained a sufficient number of cells for the enzyme assay.
The mycoplasma was removed in the test group by adding 100 μl of MRA (ICN Biomedicals Inc., Costa Mesa, CA) for each 10 ml of medium Ham-F10 with 10% FCS. The cultures were maintained without handling for 10 days in an incubator at 37 °C. In order to confirm decontamination, the mycoplasma detection test was performed for a second time in this group, prior to fibroblast collection. The cultures were held in physical isolation during handling in the laminar flow chamber and while kept in the incubator. After two weeks of the mycoplasma detection test, the fibroblasts were then removed from the flask with trypsin-EDTA solution (Cultilab Mat. Cult. Cel. Ltda, Campinas, SP, Brazil) and washed three times with PBS buffer. After centrifugation at 2000 x g for 10 minutes at 4°C (Hitachi, Himac CR 21E), the pellet containing the cells was kept frozen at -80 °C until required for protein content determination and enzyme activity assay. Protein was determined using the method described by Lowry (10).
Lysosomal enzyme activity in the cultured cells was assayed as previously described, using the appropriate substrates. Arylsulphatase B (4-nitrocatecol sulphate); b-galactosidase (4-methylumbelliferyl-b-D-galactoside); hexosaminidase A as a % of total hexosaminidase (4- methylumbelliferyl-b-D-acetylglucosamine-6-sulphate) and a-glucosidase (4-methylumbelliferyl-b-D-glucoside) (2, 8, 12, 14). The results are reported as means ± standard deviation. Mean variables were compared by oneway analysis of variance (ANOVA) followed by the Tukey test, when significant. Statistical analysis was carried out using the SPSS/PC + statistics package, with the level of significance set at p < 0.05.
In Table 1, we observed that, both in the cont 0 and the test 0 groups (fibroblast cultures with and without mycoplasma contamination), the measurement of the level of activity of lysosomal hydrolases remained below the limit considered normal in our laboratory, which confirms that these cultures are in fact cultures of affected individuals. When we compared the activity of the lysosomal enzymes in contamination-free fibroblast cultures (cont 0) with that of the same enzymes in contaminated cultures (test 0), we observed (Table 1) that in three of the four hydrolases studied there were no significant differences between the groups. Only the enzyme arylsulphatase B significantly increased its activity in the test 0 group in relation to the cont 0 group (p < 0.001).
Table 1. Lysosomal hydrolase activities in fibroblast cultures from patients with lysosomal diseasesa
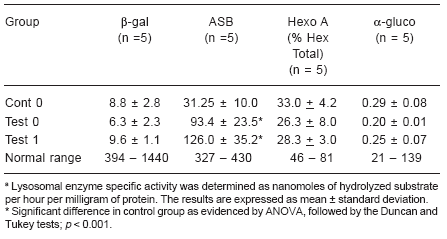
After treatment with the MRA for ten days, the detection test proved that these cultures were free of mycoplasma. New measurements of activity were then conducted on the four hydrolases. We observed that there had been no alteration in the measurement of enzymatic activity before (test 0) and after the MRA addition (test 1) in any of the enzymes studied. These results are illustrated in Table 1. The enzymatic activity in contamination- free cultures (cont 0) was compared with that of the MRA-treated cultures (test 1). The a-glucosidase, b-galactosidase and hexosaminidase A, maintained the same activity in the two groups analyzed (Table 1). However, the level of activity of the arylsulphatase B enzyme (Table 1 and Figure 2) significantly increased in the presence of the MRA (p < 0.001). We performed a curve (data not shown), using three concentrations of MRA added to the assay (50 μl, 100 μl and 150 μl), and then we observed that this agent stimulates the arylsulphatase B activity in a dose response effect.
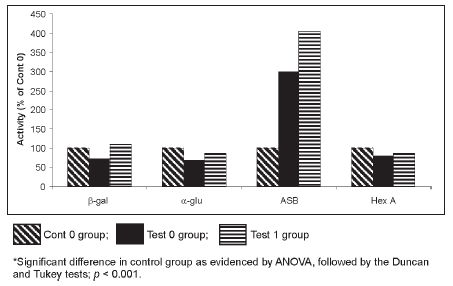
Figure 2. Fibroblast lysosomal hydrolase activities (% of Cont 0 group) from patients with lysosomal diseases.
As regards the contaminated cultures (test 0), we did not observe any significant alteration in the results of the measurement of the enzymatic activity when the MRA was added to the cultures (test 1) in the four hydrolases we studied.
It is possible that the increase in the level of activity of the arylsulphatase B enzyme, both in the presence of the mycoplasma and of the MRA could be confused with the values found in individual heterozygotes for the mucopolysaccharidosis type VI. In this case, in order to establish a precise diagnosis, it would be necessary to correlate the measurement of the enzymatic activity with the concentration and pattern of excreted urinary glycosaminoglycans as well as with the patient's clinical symptoms.
The increase in the measured levels of the lysosomal hydrolases in the presence of the mycoplasma was described in the 70´s by Gabridge et al (5). These authors described an increase in the activity of the b-glucuronidase enzyme in fibroblast cultures of mice in the presence of Mycoplasma fermentans.
The mechanism by which the contamination by mycoplasma causes the increase of the enzymatic activity in cellular cultures is not well understood. It is known that contamination by mycoplasma can lead to profound interference in lysosomal vacuoles. Studies on feline fibroblasts demonstrated that the mycoplasma is moved by phagocytosis to the insides of the lysosomal vacuoles, but, notwithstanding this, complete degradation of the mycoplasma does not occur. In the attempt to degrade the mycoplasma, many secondary lysosomes are accumulated (9). This accumulation may be one of the causes of the increase of the enzymatic activity.
On the other hand, the enzymes themselves, which are present inside the mycoplasmas, might be contributing to the increase in this activity. Reports are found in the literature stating that the mycoplasmas in cellular cultures contribute with isoenzymes that may cloud the research and confuse the results of the biochemical diagnosis (11).
The reason why the arylsulphatase B enzyme behaves differently from the others is not clear to us. There are reports in the literature on the antagonic behavior of the acid phosphatase and b-glucuronidase enzymes when exposed to the mycoplasma which suggest that this may be caused by the differences in half-life of these proteins (5).
We believe that, although all the enzymes studied are lysosomal hydrolases, the behavior pattern may be related to specific aspects of each hydrolase. In summary, the contamination by mycoplasma and the use of the MRA in the fibroblast cultures, interfered in the measurement of the activity of one of the four hydrolases we studied. This result means that biochemical diagnostic should be treated with great care due to this element of doubt. Therefore, to avoid dubious results, it is essential that mycoplasma detection be part of the routine and that, in those cultures which might be contaminated, (but which cannot be discarded), the antimicrobial MRA should be used with care and conscientiously. In this way, the patient is assured a faithful laboratorial diagnosis.
Acknowledgements: This work was supported by GPPGHCPA, CNPq and FAPERGS.
1. Ballabio A, Gieselmann V. Lysosomal disorders: from storage to cellular damage. Biochem Biophys Acta 2009; 1793: 684-96. [ Links ]
2. Butterworth J, Broadhead DM. Diagnosis of Pompe's disease in cultured skin fibroblasts and primary amniotic fluid cells using 4-methylumbelliferyl-a-glucopyranoside as substrate. Clin Chim Acta 1977; 78: 335-42. [ Links ]
3. Chen TR. In situ detection of mycoplasma contamination in cell cultures by Fluorescent Hoechst 33258 stain. Exp Cell Res 1977; 104: 255-62. [ Links ]
4. Coelho JC, Giugliani R. Fibroblasts of skin fragments as a tool for the investigation of genetic diseases: technical recommendations. Gen Mol Biol 2000; 23: 269-71. [ Links ]
5. Gabridge MG, Yip D-M, Hedges K. Levels of lysosomal enzymes in tissues of mice infected with Mycoplasma fermentans. Infec Immun 1975; 12: 233-9. [ Links ]
6. Gopalkrishna V, Verma H, Kumbhar NS, Tomar RS, Patil PR. Detection of Mycoplasma species in cell culture by PCR and RFLP based method: effect of BM-cyclin to cure infections. Indian J Med Microbiol 2007; 25: 364-8. [ Links ]
7. Harlin H, Gajewski TF. Diagnosis and treatment of mycoplasma- contaminated cell cultures. Curr Protocol Cytom 2008; 43: A.3C.1-A.3C.7. [ Links ]
8. Kresse H, von Figura K, Klein U, Glass J, Paschke E, Pohlmann R. Enzymatic diagnosis of the genetic mucopolyssaccharide storage disorders. Methods Enzymol 1982; 83: 553-9. [ Links ]
9. Larsson E, Brunk UT. Tem and Sem findings in cat fibroblasts cultivated in vitro with and without mycoplasma. Acta Path Microbial Scand 1981; 89: 0-15. [ Links ]
10. Lowry OH, Rosebrouch NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 1951; 193: 265-75. [ Links ]
11. McGarrity GJ, Vanaman V, Sarama J. Cytogenetic effects of mycoplasma infection of cell cultures: a review. In vitro 1984; 20: 1-18. [ Links ]
12. Singer JD, Cotlier E, Krimmer R. Hexosaminidase A in tears and saliva for rapid identification of Tay-Sachs disease and its carriers. Lancet 1973; 2: 1116-9. [ Links ]
13. Souza FT, Sostruznik L, Casagrande RS, Maciel de KJC, Giugliani R, Coelho JC. Comparison of the measurement of lysosomal hydrolase activity in mycoplasma-contaminated and non-contaminated human fibroblast cultures treated with mycoplasma removal agent. Clin Biochem 2007; 40: 521-5. [ Links ]
14. Suzuki K. Globoide cell leukodystrophy (Krabbe disease) and GM1 gangliosidosis. En: Glew RH, Peter SP, editors. Practical enzymology of the sphingolipidoses. New York, Alan R. Liss, 1977: p. 101-36. [ Links ]
15. Zhao H, Dreses-Werringloer U, Davies P, Marambaud P. Amyloid-beta peptide degradation in cell cultures by mycoplasma contaminants. BMC Res Notes 2008; 1: 38. [ Links ]
Recibido: 16/12/08
Aceptado: 15/11/09














