Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista argentina de microbiología
versión impresa ISSN 0325-7541versión On-line ISSN 1851-7617
Rev. argent. microbiol. v.42 n.2 Ciudad Autónoma de Buenos Aires abr./jun. 2010
MICROBIOLOGÍA INDUSTRIAL Y AMBIENTAL
Isolation of Leptospira interrogans from suburban rats in Tandil, Buenos Aires, Argentina
E. Scialfa1 *, J. Bolpe1, J. C. Bardón2, G. Ridao3, J. Gentile4, O. Gallicchio1
1División Zoonosis Rurales, España 770, (7300) Azul, Provincia de Buenos Aires;
2Laboratorio Azul SA, Provincia de Buenos Aires;
3Laboratorio de Patología, San Martín 778, (7300) Azul, Provincia de Buenos Aires;
4Servicio de Infectología del Hospital Santamarina, Paz 1430, (7000)Tandil, Provincia de Buenos Aires, Argentina.
*Correspondence. E-mail: exequielscialfa@yahoo.com.ar
Abstract
The main objective of this study was to investigate the role of wild rodents as Leptospira spp. reservoirs in a suburban area of Tandil city, Buenos Aires province (Argentina), where a person had died due to pulmonary leptospirosis. The specific objectives were: to estimate the rodent density near the patient's home, to determine the serological prevalence and isolation of leptospirosis from wild rodents, and to identify the isolated strains. The area examined was a suburban neighbourhood in Tandil near the Langueyú stream, where the patient's house is located. Rattus norvegicus were trapped on the stream banks during two nights and a high capture rate (70%), was obtained. All rats (42) were examined serologically by the microscopic agglutination test (MAT), and 22 of them (52.3%) reacted with Leptospira serovars Castellonis, Canicola, Grippotyphosa, Icterohaemorrhagiae and Hebdomadis at a titer of 1:50. The kidneys from 25 animals were cultured, and 24 isolates of L. interrogans (96%) were obtained. The isolated strains were identified as Icterohaemorrhagiae serogroup by MAT performed with rabbit hyperimmune reference sera. These findings showed a high density of suburban rodents highly infected with pathogenic leptospira, sharing environment in close contact with humans with evidence of leptospiral disease.
Key words: Leptospirosis; R. norvegicus; Transmission to humans
Resumen
Aislamiento de Leptospira interrogans de ratas suburbanas de Tandil, Buenos Aires, Argentina. El objetivo general de este estudio fue investigar el papel de los roedores silvestres como reservorios de leptospiras en área suburbana de la ciudad de Tandil (Buenos Aires, Argentina), a raíz de un caso humano fatal de leptospirosis. Los objetivos específicos fueron estimar la densidad de roedores en el peridomicilio del paciente, determinar entre éstos la tasa de positividad a leptospirosis por serología, aislar dicho microorganismo e identificar las cepas aisladas. El área estudiada fue un barrio suburbano de Tandil próximo al arroyo Langueyú, donde residía el paciente. Se atraparon ejemplares de Rattus norvegicus sobre la orilla del arroyo durante dos noches, con una alta tasa de captura (70%). En las 42 ratas atrapadas se examinó la serología por el test de microaglutinación (TMA); 22 de ellas (52,3%) reaccionaron con los serovares Castellonis, Canicola, Grippotyphosa, Icterohaemorrhagiae y Hebdomadis, a títulos de 1:50. El cultivo de tejido renal de 25 animales permitió obtener 24 aislamientos de L. interrogans (96%). Las cepas aisladas fueron identificadas como serogrupo Icterohaemorrhagiae mediante TMA utilizando suero hiperinmune de conejo. Estos hallazgos muestran una elevada densidad de roedores infectados con leptospiras patógenas en el área suburbana de la ciudad de Tandil, en estrecho contacto con población humana con evidencia de leptospirosis.
Palabras clave: Leptospirosis; R. norvegicus; Transmisión al hombre
Leptospirosis is a zoonotic disease of global importance that occurs in urban and rural settings, which causes endemic and epidemic illness (3, 4, 8, 10), including pulmonary hemorrhage and death (12, 13).
A wide variety of animals may serve as sources of human infection. The relative importance of a given species varies depending on the area, the population density of the species, the type and character of human housing, and the occupational and leisure activities of the local residents. In some instances, a wild animal species presents little direct risk to human health but indirectly causes human illness by transmitting leptospirosis to an intermediary domestic animal species. Rodents are the most important wild mammal source of human leptospirosis (3, 5, 9). Transmission to humans results from exposure to the urine of infected animals by direct contact or through contaminated water (2). Contact with rodents and water sources are significant factors, particularly in flood periods (3, 4, 8).
In May 2007 (fall season), a 22-year-old male patient died after being diagnosed with leptospirosis. He reported previous contact with flood water in a suburban neighbourhood in Tandil, a city located in the center of Buenos Aires province (Argentina). He presented a non-specific febrile illness with myalgia, jaundice, thrombocitopenia, leukocytosis (with a left shift), a very high creatine phosphokinase (CPK) level, dysnea and bilateral infiltration of the lungs on the chest X-ray. He later developed respiratory failure, coagulopathy and renal dysfunction; and died 15 days after onset of illness by respiratory distress due to pulmonary hemorrhage. The definitive diagnosis of leptospirosis was suggested by a 4-fold rise in titer (1:400) and a positive result of the microscopic agglutination test (MAT) with 10 serovars during the second week of disease and previous negative result of the microscopic agglutination test at the onset of illness.
Due to this human case, our main objective was to investigate the role of wild rodents as leptospira reservoirs in a suburban area of Tandil city. The specific objectives were to determine the rodent density near the patient's place of residence, to estimate the positivity rate of serology and isolation among the Rattus norvegicus trapped and finally, the identification of the strains isolated.
The area examined was a suburban neighbourhood in Tandil near the Langueyú stream, where the patient's house is located. Wild rodents were captured with 30 Tomahawk tramps distributed over the stream banks during two nights. The captured rats were euthanized by ether inhalation, and necropsy was performed under biosafety conditions (1). Blood samples were obtained by heart puncture of the captured animals, and kidney tissue samples were aseptically collected for culture isolation.
The MAT with live antigen was used for the diagnosis of leptospirosis in humans and rodents. The MAT was performed with a battery of 10 leptospire serovars (Leptospira Castellonis, Leptospira Canicola, Leptospira Grippotyphosa, Leptospira Hardjo, Leptospira Hebdomadis, Leptospira Icterohaemorrhagiae, Leptospira Pomona, Leptospira Pyrogenes, Leptospira Wolfi, Leptospira Tarassovi) maintained in EMJH medium (2). A titer of 1:50 was considered positive. Kidney tissue was suspended in a transport medium (buffered solution containing 200 mg/ml of 5-fluoracil). Dilutions 1:10 and 1:100 were made with sterile buffered saline, and 0.1 ml was inoculated into EMJH medium, and incubated at 28 °C during 90 days (6). Leptospiral growth was weekly monitored using dark field microscopy. Differentiation of pathogenic and saprophytic Leptospira was done by culture in media with and without copper sulfate, and growth at different temperatures (7). An additional pathogenicity test was carried out by intraperitoneal inoculation of young hamsters (50-60 g), using 0.5 ml of the fluid culture isolation. Isolated strains were identified by MAT performed with rabbit hyperimmune sera prepared in the following reference centers: Istituto Superiore di Sanità di Roma (Italy), and the Royal Tropical Institute in Amsterdam, The Netherlands (OIE Reference Laboratory for leptospirosis). Serovars representative of 8 Leptospira serogroups were used: L. Canicola, L. Icterohaemorrhagiae, L. Pomona, L. Grippotyphosa, L. Tarassovi, L. Hardjo, L. Castellonis and L. Bratislava.
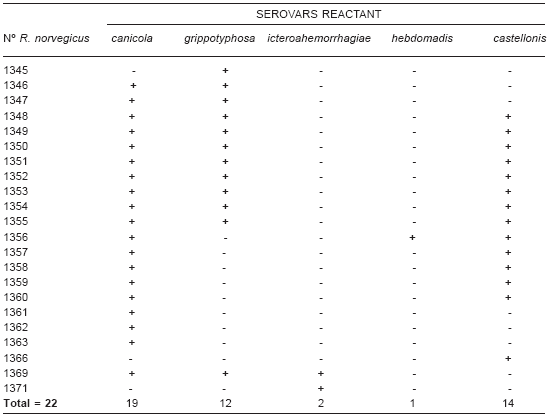
Over a period of 2 nights, 42 R. norvegicus -21 females and 21 males- were captured (70% capture rate). All animals were tested by MAT, and 22 (52.3%) were positive with titers of 1:50 (9/21 females and 13/21 males) with the results indicated (Table 1). Coagglutination was evident in 15/22 rats, with predominance of Castellonis-Canicola serovars.
Table1. Distribution of seropositive rodents according to serovars.
Kidneys from 25 rats were cultured, and 24 isolates were obtained (96%), with only twelve of them showing serological evidence of leptospiral infection (concordance 48%) (Table 2). Using differential media (with and without copper sulfate), growth at different temperatures and a pathogenicity test, the isolated strains were identified as L. interrogans. There was no leptospiral growth in medium containing copper sulfate during the 30 day- incubation period, and at 15 °C of incubation temperature the growth of the isolated strain was low. All the leptospiral isolations were identified as Icterohaemorrhagiae serogroup by MAT performed with rabbit hyperimmune reference sera.
Table 2. Comparison of results of bacteriological isolation and microagglutination test.

R. norvegicus is the most frequently captured species from suburban environments; however, the high capture obtained is not habitual, showing an expanding rat population in the Langueyú stream area. The high seropositivity in R. norvegicus was also consistent with previous studies (4, 11). Wild rodents infected with leptospira show a variable antibody response and, occasionally, a low titer or undetectable levels to MAT. According to these, we detected evidence of infection by leptospira isolation, without serological evidence in 50% of the studied animals.
The isolation rate obtained from kidney tissue (96%) was higher than that in previous reports (5, 11, 13, 14).
In Argentina, the Icterohaemorrhagiae serogroup is the most common leptospiral isolate from R. norvegicus; however, occasionally, another serogroup such as Ballum, has been found (15).
Rodent infestations in the neighborhood are able to maintain an endemic infection. The trapped wild rodents infected with L. Icterohaemorrhagiae are important carriers of highly pathogenic Leptospira, and probably have greater potential risk factors of transmission to humans.
Programs for rodent control are necessary in order to interrupt transmission of leptospirosis and effectively reduce the occurrence of human cases.
Acknowledgements: He thank Dr. Ariel Koval at Biogenesis Bago SA Laboratory for his professional work in sending us their laboratory test results (identification of isolates by MAT performed with rabbit hyperimmune reference sera).
1. Adala R, Bolpe J, Contarelli JM, Gonzalez Ayala S, Iucci C, Marchan H, et al. Guía para el Tratamiento, Control y Prevención de Hantavirosis. Ministerio de Salud de la Provincia de Buenos Aires, 2003. [ Links ]
2. Faine S. Leptospira and leptospirosis. Boca Raton, CRC Press, 1994, p. 147-9. [ Links ]
3. Jansen A, Schôneberg I, Frank C, Alpers K, Schneider T, Stark K. Leptospirosis in Germany, 1962-2003. Emerg Infect Dis 2005; 11: 1048-53. [ Links ]
4. Johnson MAS, Smith H, Joseph P, Gilman RH, Bautista CT, Campos KJ, et al. Environmental exposure and leptospirosis, Perú. Emerg Infect Dis 2004; 10: 1016-22. [ Links ]
5. Martin UM, Sensevy A, Colombo J, Tramontin V. Leptospirosis en la provincia de Santa Fe, descripción epidemiológica, clínica y socioeconómica. Medicina (Buenos Aires) 2002; 62: 164-8. [ Links ]
6. OPS/OMS. Manual de Métodos para el Diagnóstico de Laboratorio de la Leptospirosis. Nota Técnica N° 30, 1985, p. 9-20. [ Links ]
7. Russell CJ, Virginia GH. Differentiation of pathogenic and saprophytic leptospires. Growth at low temperatures. J Bacteriol 1967; 94: 27-31. [ Links ]
8. Scialfa E, Gallicchio O, Benitez M. Leptospirosis humana y animal en un barrio suburbano de alto riesgo en el partido de Azul, Buenos Aires, Año 2000. IV Congreso Argentino de Zoonosis, 2004, resumen A15 p. 21, Buenos Aires, Argentina. [ Links ]
9. Scialfa E, Gallicchio O, Benitez M. Leptospirosis humana. Incidencia en la provincia de Buenos Aires, periodo 2003- 2005. Rev Col Vet Prov Bs As 2007; 38: 38-40. [ Links ]
10. Scialfa E, Gallicchio O, Cerruti S, Zungri R. Brote de leptospirosis humana en trabajadores rurales de la localidad de Magdalena: posible asociación de los bovinos como fuente de infección. Rev Col Vet Prov Bs As 2004; 28: 53-4. [ Links ]
11. Scialfa E, Ribicich M, Amiotti P, Bolpe J, Gallicchio O, Vizcay R, et al. Estudio de leptospirosis en animales silvestres de las provincias de Buenos Aires y Neuquen, Año 2005. I Congreso Panamericano de Zoonosis, V Congreso Argentino de Zoonosis y II Congreso Bonaerense de Zoonosis, 2006, suplemento 3, p. 305, Buenos Aires, Argentina. [ Links ]
12. Seijo A, Argento E, Dorta de Mazzonelli G, Dragui MG, Mazzonelli J, Stiebel C, et al. Comisión Científica sobre Leptospirosis de la República Argentina (CCLA). Informe sobre Leptospirosis en la República Argentina. Fundación Mundo Sano, editor, 2° ed. 2006, Buenos Aires, Argentina. [ Links ]
13. Seijo A, Coto H, San Juan J, Videla J, Deodato B, Cernigoi B, et al. Distrés respiratorio debido a hemorragia pulmonar por leptospirosis. Medicina (Buenos Aires) 2002; 62: 135-40. [ Links ]
14. Vado-Solís I, Cárdenas-Marrufo MF, Jiménez-Delgadillo B, Alzina-López A, Laviada-Molina H, Suarez-Solís V, et al. Clinical-epidemiological study of leptospirosis in humans and reservoirs in Yucatán, México. Rev Inst Med Trop São Paulo 2002; 44: 335-40. [ Links ]
15. Vanasco NB, Sequeira MD, Sequeira G, Tarabla HD. Associations between leptospiral infection and seropositivity in rodents and environmental characteristics in Argentina. Prev Vet Med 2003; 60: 227-35. [ Links ]
Recibido: 10/11/09
Aceptado: 17/02/10














