Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista argentina de microbiología
versión impresa ISSN 0325-7541
Rev. argent. microbiol. vol.42 no.4 Ciudad Autónoma de Buenos Aires oct./dic. 2010
CLINICAL MICROBIOLOGY AND INFECTIOUS DISEASES
AIDS pacient's long-term battle with multiply recurrent tuberculosis: reinfection or reactivation?
A. Reniero1, M. Beltrán1, I. N. de Kantor2, V. Ritacco3*
1Hospital Municipal de San Isidro, Santa Fe 431, (1642) San Isidro;
2PAHO/WHO consultant, Av Libertador 7504, (1429) Buenos Aires;
3INEI ANLIS "Carlos Malbrán", Vélez Sarsfield 563, (1281) Buenos Aires, Argentina.
*Correspondence. E-mail: vritacco@anlis.gov.ar
ABSTRACT
The advent of Mycobacterium tuberculosis strain genotyping has allowed differentiation between disease relapse and exogenous re-infection. We report here a remarkable case of multiply recurrent tuberculosis in a patient living with HIV. Between 1995 and 2009, a young HIV-infected intravenous drug user, who was reluctant to comply with anti-retroviral treatment, underwent at least five tuberculosis episodes caused by three distinct M. tuberculosis strains sharply differentiated by drug susceptibility profile, genotype and infectious source. Eventually, the patient died during a relapse of tuberculosis due to a notorious multidrug-resistant outbreak-strain, which infected him during a prolonged hospitalization in the epicentre of such outbreak. Whether recurrent tuberculosis is due to a new infection or to reactivation of a previous one is a century-long controversial question. In our patient, both conditions alternated throughout his 15 years of living with HIV. Cases such as this might not be exceptional in certain underprivileged suburban areas of Argentina and should raise concern over three pending issues in tuberculosis control policies, namely secondary preventing therapy, institutional infection control and patient follow-up throughout the health network system.
Key words: Tuberculosis; Recurrence; Relapse; Reinfection; AIDS; HIV
RESUMEN
Larga batalla de un paciente con sida y tuberculosis recurrente: ¿reinfección o reactivación? La genotipificación de aislamientos clínicos de Mycobacterium tuberculosis permite diferenciar entre recaída y reinfección exógena. Presentamos un notable caso de tuberculosis recurrente asociado a sida. Entre 1995 y 2009, un usuario de drogas ilegales por vía endovenosa, con infección por el VIH pero reacio al tratamiento antirretroviral, sufrió cinco episodios de tuberculosis causados por tres cepas de M. tuberculosis. Las cepas se diferenciaron por los genotipos, los perfiles de sensibilidad a antibióticos y las fuentes de infección. El paciente murió de tuberculosis por una cepa multirresistente, responsable de un extenso brote en Argentina, que lo infectó durante una prolongada internación en el hospital que fue el epicentro de dicho brote. La controversia sobre el origen de la tuberculosis recurrente -reinfección o reactivación- lleva ya un siglo y continúa vigente. En el caso que aquí se describe, las dos situaciones se alternaron a lo largo de los 15 años de lucha contra el sida que atravesó este paciente. Esta situación puede no ser excepcional en áreas suburbanas desfavorecidas de Argentina, y pone en evidencia tres temas insuficientemente atendidos en nuestro medio: el tratamiento preventivo de la tuberculosis secundaria, el control de su transmisión en hospitales y cárceles, y el seguimiento de los pacientes a través del sistema de salud.
Palabras clave: Tuberculosis; Recurrencia; Recaída; Reinfección; Sida; VIH
Molecular strain typing has made a major contribution to our understanding of tuberculosis pathogenesis by shedding light on the pathways underlying tuberculosis recurrence (2, 4, 7). Unexpected high rates of tuberculosis re-infection were demonstrated by Mycobacterium tuberculosis genotyping in settings with high burden of the disease, particularly in association with Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome (HIV/AIDS) (3, 5, 8) . Herein we describe a remarkable case of multiply recurrent tuberculosis documented in a patient who lived with HIV/AIDS between 1995 and 2009.
In November 1994, after 15 years of alcohol addiction and intravenous use of illegal drugs, a 29 year-old man resolved to seek advice at our HIV clinic. In that first consultation, the patient was completely asymptomatic and seronegative for HIV, as found one year before, at admission into a state prison.
In January 1995, our patient presented with a fever, poly-adenopathies and chest pain. Tuberculosis was diagnosed on the basis of an abnormal chest X-ray, a 20 mm tuberculin skin test and a sputum smear positive for acid fast bacilli. Tubercle bacilli grown upon culture were resistant to isoniazid and susceptible to rifampicin, streptomycin, ethambutol and pyrazinamide. The isolate shared identical IS6110 restriction fragment length polymorphism DNA fingerprint (11), spoligotype (6) and drug susceptibility patterns (12), as performed according with international standards, with the strain identified one year before in the sputum of one of his prison inmates.
Isoniazid resistance remained unnoticed to the clinicians and our patient received a combination treatment consisting in a 2-month initial phase of isoniazid, rifampicin, pyrazinamide and ethambutol and an 8-month continuation phase of isoniazid and rifampicin. In May 1995, HIV seroconversion was documented by enzyme immunoassay and confirmed by Western Blot. Upon completion of treatment for tuberculosis, in August 1995, the patient was asymptomatic and both acid fast bacilli smear examination and mycobacterial culture were negative (Figure 1).
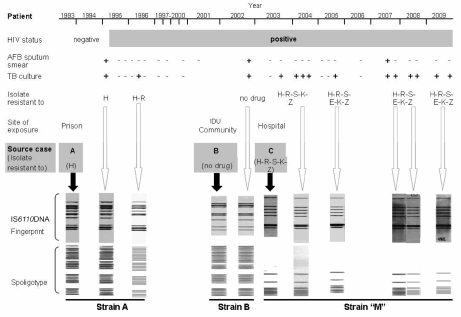
Chronological sequence of relevant microbiological, epidemiological and genotyping features in the study case from diagnosis to death, occurred in December 2009. TB: tuberculosis. AFB: acid fast bacilli. H. isoniazid, R: rifampicin, S: streptomycin, E: ethambutol, K. kanamycin, Z: pyrazinamide. IDU: intravenous drug user. White arrows point to genotypes of successive isolates of the study case; black arrows point to genotypes of source cases.
Acid fast bacilli smear-positive pulmonary tuberculosis relapsed in March 1996. The same strain of the initial episode had now become resistant to rifampicin and lost one IS6110 band in the restriction fragment length polymorphism pattern. The patient was then prescribed a scheme including ethambutol, streptomycin and ofloxacin, and treatment for tuberculosis was again completed until culture became negative. Sputum smear and culture were repeatedly negative in 1998. Thereafter the patient did not seek assistance at our clinic for a long period, during which he frequently was taken under police detention or to prison.
In September 2002 the patient returned with clinical and radiological signs that were typical of pulmonary tuberculosis. This time, the isolated bacilli were fully susceptible to drugs and shared identical IS6110 restriction fragment length polymorphism pattern with a M. tuberculosis strain isolated six months before from one of his drug-user partners in the community. At that time, we submitted the patient to a standard directly observed treatment short-course regimen for fully susceptible tuberculosis. Due to a worsening of his clinical condition shortly after initiating treatment, the patient was submitted to a prolonged hospitalization in the Muñiz Hospital, the epicenter of an ongoing tuberculosis epidemic caused by a multidrug-resistant strain named strain "M" (9, 10). Upon treatment completion, bacteriological cure from fully susceptible tuberculosis, weight gain and amelioration of clinical status, our patient was discharged from the Muñiz Hospital.
A few months later, he sought again ambulatory assistance at our clinic. Once more, active tuberculosis was diagnosed and this time disease was due to the outbreak multidrug resistant strain "M", which had been transmitted to him by a ward inmate at the Muñiz Hospital. Since then, our patient, who was never compliant with antiretroviral treatment, was again repeatedly detained or confined in prison. While not in jail, he was hospitalized alternatively in our health centre or in the Muñiz Hospital and treated with second-line tuberculosis treatment schemes including cycloserine, ethionamide, ofloxacin and ethambutol. Periods of bacteriological activity and clinical signs of pulmonary tuberculosis alternated with periods of bacteriological and clinical tuberculosis remission, while his general clinical status was progressively deteriorating. At the last stages of disease, positive sputum cultures on solid medium were disgonic and yielded only a few colonies. The "M" strain was identified in every positive culture from July 2003 until December 2009, when he died in the Muñiz Hospital from consumption and extensive pulmonary destruction.
Whether recurrent tuberculosis is due to a newly acquired infection or to reactivation of a previous one is a century-long controversial question (4). In our case, both conditions alternated throughout the 15 years of the patient living with HIV/AIDS. There is ground to assume that our patient's reluctance to comply with antiretroviral treatment has played a major role in tuberculosis recurrence since antiretroviral therapy has proved to decrease substantially its likelihood (1). Still, cases such as the one described here might not be exceptional in underprivileged suburban areas of Argentina because in such settings prolonged access to patient follow-up and, particularly, to genotype documentation, are utterly unusual.
A particular challenge to the clinical management of this case was posed by the sharp differences in drug susceptibility profiles observed in the successive infecting strains ( Figure 1). This issue has critical therapeutic implications that are particularly onerous in setting like ours, where turnaround times of mycobacterial drug susceptibility testing are still disappointingly long. While under supervision, the patient was compliant with tuberculosis treatment and, soon after a proper treatment was installed, his sputum smear became readily negative for acid fast bacilli, suggesting that this case is unlikely to have been highly infectious during his prolonged periods of active tuberculosis. To our knowledge, there was no evidence of household contacts developing disease throughout the whole study period. However, no proper contact tracing was performed and infection transmission cannot be discarded.
The unfortunate case described here is a conspicuous example of health care mismanagement. Paradoxically, tuberculosis continues being the biggest killer of people living with HIV/AIDS even though well-known interventions are widely available for its prevention and treatment (2). Cases such as this should raise concern over three pending issues regarding the control of tuberculosis in our country, namely secondary preventing therapy, institutional infection control and patient follow-up throughout the health network system, this latter involving the penitentiary system. Such interventions are most urgently needed in settings where high loads of tuberculosis and HIV/AIDS converge.
Acknowledgement: VR is a scientist of CONICET, Argentina. The work was partially financed by grant PAE 37245/PICT 02323 of the Argentinean Ministry of Science (MinCyT).
1. Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray A, et al. Effect of initiating antiretroviral therapy during tuberculosis treatment in HIV-infected individuals: results of a randomized controlled trial in TB-HIV co-infected patients in South Africa (SAPiT Study). N Engl J Med 2010; 362: 697-706. [ Links ]
2. Chaisson RE, Churchyard GJ. Recurrent tuberculosis: relapse, reinfection, and HIV. J Infect Dis 2010; 201: 653-5. [ Links ]
3. Charalambous S, Grant AD, Moloi V, Warren R, Day JH, van Helden P, et al. Contribution of reinfection to recurrent tuberculosis in South African gold miners. Int J Tuberc Lung Dis 2008; 12: 942-8. [ Links ]
4. Chiang CY, Riley LW. Exogenous reinfection in tuberculosis. Lancet Infect Dis 2005; 5: 629-36. [ Links ]
5. Crampin AC, Mwaungulu JN, Mwaungulu FD, Mwafulirwa DT, Munthali K, Floyd S, et al. Recurrent TB: relapse or reinfection? The effect of HIV in a general population cohort in Malawi. AIDS. 2010; 24: 417-26. [ Links ]
6. Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, et al. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol 1997; 35: 907-14. [ Links ]
7. Mathema B, Kurepina N, Fallows D, Kreiswirth BN. Lessons from molecular epidemiology and comparative genomics. Semin Respir Crit Care Med 2008; 29: 467-80. [ Links ]
8. Narayanan S, Swaminathan S, Supply P, Shanmugam S, Narendran G, Hari L, et al. Impact of HIV infection on the recurrence of tuberculosis in South India. J Infect Dis 2010; 201: 691-703. [ Links ]
9. Palmero D, Ritacco V, Ambroggi M, Marcela N, Barrera L, Capone L, et al. Multidrug-resistant tuberculosis in HIV-negative patients, Buenos Aires, Argentina. Emerg Infect Dis 2003; 9: 965-9. [ Links ]
10. Ritacco V, Di Lonardo M, Reniero A, Ambroggi M, Barrera L, Dambrosi A, et al. Nosocomial spread of Human Immunodeficiency Virus-related multidrug-resistant tuberculosis in Buenos Aires. J Infect Dis 1997; 176: 637-42. [ Links ]
11. van Embden JD, Cave MD, Crawford JT, Dale JW, Eisenach KD, Gicquel B, et al. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol 1993; 31: 406-9. [ Links ]
12. World Health Organization. Laboratory services in tuberculosis control. WHO/TUBERCULOSIS/98.258. [ Links ]
Recibido: 29/06/10
Aceptado: 30/08/10














