Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista argentina de microbiología
Print version ISSN 0325-7541
Rev. argent. microbiol. vol.43 no.2 Ciudad Autónoma de Buenos Aires June 2011
MICROBIOLOGÍA INDUSTRIAL Y AMBIENTAL
Chemotactic responses to gas oil of Halomonas spp. strains isolated from saline environments in Argentina
Sebastián D´Ippólito, Rosana E. de Castro, Karina Herrera Seitz*
Instituto de Investigaciones Biológicas, Universidad Nacional de Mar del Plata. Funes 3250 4to Nivel, Casilla de Correo 1245, Mar del Plata (7600) Argentina.
Correspondence. E-mail: khseitz@mdp.edu.ar
ABSTRACT
In this study, two halophilic bacterial strains isolated from saline habitats in Argentina grew in the presence of gas oil. They were identified as Halomonas spp. and Nesterenkonia sp. by 16S ribosomal RNA sequencing. Chemotaxis towards gas oil was observed in Halomonas spp. by using swimming assays.
Keywords: Halophiles; Halomonas; Hydrocarbon degradation; Chemotaxis
RESUMEN
Respuesta quimiotáctica hacia gas oil de cepas de Halomonas spp. aisladas de ambientes salinos de Argentina. En el presente trabajo se aislaron dos cepas bacterianas halofílicas a partir de muestras obtenidas en ambientes salinos de Argentina, que crecieron en presencia de gasoil como única fuente de carbono. Las cepas aisladas se identificaron como Halomonas spp. y Nesterenkonia sp. mediante secuenciación del gen del ARN ribosomal 16S. En ensayos de swimming, las cepas del genero Halomonas spp. mostraron una respuesta quimiotáctica hacia el gas oil.
Palabras clave: Halófilos; Halomonas; Degradación de hidrocarburos; Quimiotaxis
Bioremediation takes advantage of the ability of microorganisms to detoxify natural environments by degrading and using organic contaminants such as carbon and energy sources. Similarly to what happens in other ecosystems, saline/hypersaline environments are affected by contamination as a result of industrial effluents with high salt concentrations that are commonly produced by several industries like the leather and hydrocarbon (HC) industries. Since halophilic microorganisms are physiologically adapted to grow at high salt concentrations, they are expected to be more efficient for contaminant removal/ degradation than non-halophiles. Thus, halophiles represent a valuable resource to remediate contaminated saline environments and effluents.
During the last decade, biodegradation of pollutants in saline environments was demonstrated and various halophilic and halotolerant microbes responsible for such activity were identified. These include: cyanobacteria (1), the halotolerant bacteria Halomonas spp. which degrades chloroaromatic compounds and phenols (3, 7), the haloarchaeon Halobacterium sp. isolated from a HC contaminated site (7) and members of genera Haloarcula and Haloferax that have been isolated from different types of hypersaline environments including salt lakes and crystallizer ponds from solar salterns (9, 14). Recently, Tapilatu et al. (12) reported the degradation of alkane and polyaromatic HC by HC-degrading haloarchaeal strains related to genera Haloferax and Haloarcula isolated from polluted or uncontaminated environments. Several physicochemical and biological factors can affect the efficiency of bioremediation processes in natural environments and chemotaxis is one of such factors. Chemotaxis allows the cells to detect and respond to concentration gradients of different compounds. A number of bacterial strains have been described to be chemotactic towards the pollutants they are able to degrade (6, 10, 11), thus, the presence of chemotaxis is considered to be a desirable characteristic in bacteria involved in the biodegradation processes of contaminated environments (11). However, the chemotactic behaviour to HC has been poorly investigated in halophiles.
In this study, two halophilic bacterial strains were isolated and identified from saline environments in Argentina and they were tested for their ability to grow on gas oil (GO), a petroleum-derived fuel, and to respond chemotactically to this HC mix.
Two different saline habitats were examined for the occurrence of HC-metabolizing microorganisms: a salt brine located in Bahía Blanca estuary without HC contamination (38° 44' South 62° 16' West) and HC-contaminated sea water sampled from the harbor of Mar del Plata city (38° 00' South 57° 33' West). The samples obtained from the salt brine were initially grown in a saline medium containing (g/l): NaCl 100, MgSO4 7H2O 14, KCl 1.4, sodium citrate 2.2, CaCl2 2H2O 0.14; 0.05 % yeast extract (YE) (w/v) and 0.2% GO (v/v), at 37 ºC with agitation. GO is a complex mixture of HC which contains 75 % (v/v) alkanes (mainly hexadecane) and 25 % (v/v) aromatic HC (including naphtalenes and alkyl benzenes). This culture was used to inoculate (inoculation volume was 5 to 10% of the total culture) fresh medium containing only 0.025% YE (no sodium citrate) with and without GO (0.2 %) and incubated at 37 °C with agitation. Seawater samples were filtered through a 0.2 μm nitrocellulose membrane and the microorganisms retained on its surface were used to inoculate sterile seawater in the presence and absence of 0.2% GO and incubated at 25 °C for several days with agitation. Cultures grown with 0.5 % YE as the carbon and nitrogen sources were included as controls (data not shown). Cell growth was determined by measuring the optical density of the cultures at 600 nm (OD600). Figure 1 A and B shows that cell growth was higher in the cultures containing GO compared to controls without GO (doubling times based on OD600 values were ~ 25 h in both samples). Although the cell density of the cultures was rather low, particularly in that inoculated with the HC-contaminated seawater, the results were reproducible in different experiments. Even though the concentration of GO along the growth curve was not directly measured, comparison of the time courses of cell growth in the presence and absence of GO suggests that the microbes in the saline samples were able to use the HCs present in GO. Gas chromatography analysis of the culture media before and after cell growth as well as the determination of cell growth in the presence of the individual HCs will help to identify the potential substrate/s metabolized by these halophiles. When observed under the microscope, very motile rod-shaped cells were visualized in the GO-cultures from the saline samples (salt brine and sea water) and this feature facilitated the development of the chemotaxis assays. Unlike what happened in GO-containing cultures, almost no cells were detected in those without HC. To isolate the HC-degrading microorganisms, after several subcultures in the presence of GO both cultures were diluted and plated on the corresponding medium with GO and incubated until bacterial colonies were evident. Two different colonies were visualized in the salt brine sample, suggesting that at least two distinct microorganisms had been isolated. Liquid cultures from these isolates retained the ability to grow on GO (not shown) although only one of these isolates showed good motility. On the other hand, a single type of colony was isolated from the Mar del Plata harbor on GO-containing plates. To taxonomically identify the isolated halophiles, a fragment (1.3 kb) of the 16S ribosomal RNA (rRNA) gene was PCR-amplified and sequenced. With this aim, primers F43 Eco (5'-CGGAATTCCAGGCCTAACACATGCAAGTC- 3') and R1387 Eco (5'-CGGAATTCGGGCGGWGTGTACAAGGC- 3') based on conserved sequences of bacterial 16S rRNA gene were used (8). Briefly, single colonies were suspended in 50μl sterile water and boiled for 5 minutes. These cell suspensions (5-10 μl) were used as template for the PCR reactions which contained: 0.25 μmoles/l of each primer (F43Eco and R1387Eco), 0.5 mmoles/l dNTPs, 0.5U Taq DNA polymerase (P-BL, Quilmes, Argentina), 1X Taq DNA polymerase buffer and 3 mmoles/l MgCl2. Amplifications were carried out using the following temperatures: (94 °C 10 min) x 1; (95 °C 1 min, 55 °C 1 min, 72 °C 90 s) x 30, (72 °C 5 min) x 1. PCR products were fractionated on 0.8 % (w/v) agarose gels containing 0.5 μg/ml ethidium bromide and the DNA fragments of the expected size (1.3 kb) were purified and sequenced (Macrogen, Korea).

Figure 1. Growth curve of halophiles isolated from saline environments in the presence of GO. Samples taken from the saline environments were inoculated with agitation in the indicated media and temperature. (A) Salt brine sample. (B) Sea water sample. The results are representative of at least three independent experiments.
The nucleotide sequences of the amplified fragments were analyzed in Public Databases (http://rdp.cme.msu.edu and NCBI/BLAST). This allowed the identification of genus Halomonas (98 % identity to Halomonas desiderata), accession number HM454286 and Nesterenkonia (97 % identity to Nesterenkonia aethiopica and Nesterenkonia halobia) accession number HM454287 from the salt brine and genus Halomonas from the HC-contaminated sea water (97 % identity to Halomonas sp. ice-oil-302), accession number HM454288. Halomonas sp. belongs to the class gammaproteobacteria, family Halomonadacea, while Nesterenkonia sp. is included in the class Actinobacteria, order Actinomycetales, family Micrococcaceae.
The chemotactic behaviour of the halophilic bacterial strains isolated in this study was evaluated on swimming plates (13). In this assay, the attractant is added into the medium and bacteria generate and follow a gradient as they degrade and grow on the attractant. As a result a "ring of cells" is observed after incubation of the swimming plates for several hours at the indicated temperature. As shown in Figure 2 A and D, swimming rings were observed in both Halomonas spp. strains in the presence of GO indicating that they were chemotactic to the HC mix. This means that the Halomonas strains isolated in this study may have the ability not only to degrade but also to detect and actively swim towards some GO component/s ; otherwise, cells only growing on GO but not responding to it would accumulate in the site of inoculation, as observed for the Nesterenkonia sp. strain, which did not exhibit motility (data not shown). As expected, no responses were evidenced in the absence of any carbon source (Figure 2, C and F). Although chemotaxis to aromatic HC has been reported in some non-halophilic bacteria (4, 10, 11), a chemotactic behaviour of microbes to alkanes (GO and hexadecane) has only been demonstrated for the bacterium Flavimonas oryzihabitans isolated from a HC-contaminated site from Argentina (6). The Halomonas spp. strains identified in this study also showed a chemotactic response towards YE (Figure 2 B and E), casamino acids, and citrate (data not shown). To the best of our knowledge, these findings represent the first report on chemotactic behaviour in the genus Halomonas. Although the ability of soil bacteria to degrade different toxic compounds is well documented, there is comparatively limited information on the catabolic activity of halophiles on organic pollutants and their potential to remediate contaminated saline ecosystems. Microbial diversity studies of several saline environments from Argentina have been carried out in the last years (2, 5). As an example, the study of community shifts in an exploited oil field with naturally high soil salinity near Comodoro Rivadavia in Patagonia (Argentina) identified a number of halophilic genera including Halomonas, Dietzia and Alcanivorax (5). Our study describes the isolation and identification of two halophilic bacteria related to genus Halomonas spp. and Nesterenkonia sp. from two different saline ecosystems of Argentina which grew on a mixture of HC (GO) and displayed chemotaxis to these compounds (Halomonas sp.). The occurrence of Halomonas spp. in different saline habitats of Argentina suggests that this genus may predominate in saline Argentine ecosystems or that these bacteria are easily isolated due to their ability to grow under laboratory conditions in the presence of toxic compounds. These findings suggest the potential of genus Halomonas as a biological tool for the remediation of HC-contaminated saline ecosystems.
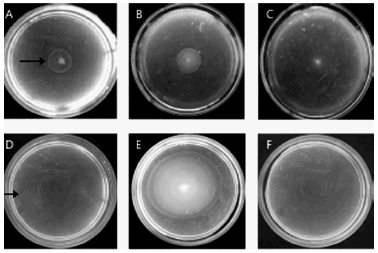
Figure 2. Determination of the chemotactic response towards GO of Halomonas spp isolated from saline environments in Argentina. Swimming plates of Halomonas spp isolated from salt brine (A, B, C) and/or sea water (D, E, F). Overnight cultures (4 μl) were inoculated in the centre of soft agar plates containing GO (A, D), 0.05 % YE (B, E) or in the absence of a carbon source (C, F). Plates were photographed after 24 h of incubation at 30 °C. Arrowheads show swimming rings. The results are representative of at least three independent experiments.
Acknowledgements: This work was supported by grants from Comisión de Investigaciones Científicas (CIC) prov. de Buenos Aires, Argentina, awarded to R. De Castro and M.K Herrera Seitz. The authors wish to acknowledge Dr C. Studdert and S. Peresutti for reading the manuscript and their helpful suggestions.
1. Abed R, Al-Thukair A, De Beer D. Bacterial diversity of a cyanobacterial mat degrading petroleum compounds at elevated salinities and temperatures. FEMS Microbiol Eco 2006; 57: 290-301. [ Links ]
2. Ferrero M, Farías M, Siñeriz F. Preliminary characterization of microbial communities in High Altitude Wetlands of Northwestern Argentina by determining terminal restriction fragment length polymorphisms. Rev Latinoam Microbiol 2004; 46: 72-80. [ Links ]
3. Garcia M, Ventosa A, Mellado E. Catabolic versatility of aromatic compound-degrading halophilic bacteria. FEMS Microbiol Ecol 2005; 54: 97-109. [ Links ]
4. Grimm A, Harwood C. Chemotaxis of Pseudomonas spp. to the polyaromatic hydrocarbon naphthalene. Appl Environ Microbiol 1997; 63: 4111-5. [ Links ]
5. Kleinsteuber S, Riis V, Fetzer I, Harms H, Müller S. Population dynamics within a microbial consortium during growth on diesel fuel in saline environments. Appl Environ Microbiol 2006; 72: 3531-42. [ Links ]
6. Lanfranconi M, Alvarez H, Studdert C. A strain isolated from gas oil-contaminated soil displays chemotaxis towards gas oil and hexadecane. Environ Microbiol 2003; 5: 1002-8. [ Links ]
7. Le Borgne S, Vazquez-Duhalt D. Biodegradation of organic pollutants by halophilic bacteria and archaea. J Mol Microbiol Biotechnol 2008; 15: 74-92. [ Links ]
8. Marchesi J, Sato T, Weightman A, Martin T, Fry J, Hiom S, Wade W. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl Environ Microbiol 1998; 64: 795-9. [ Links ]
9. Oren A. Diversity of halophilic microorganisms: environments, phylogeny, physiology, and applications. J Ind Microbiol Biotechnol 2002; 28: 56-63. [ Links ]
10. Pandey G, Chauhan A, Samanta S, Jain R. Chemotaxis of a Ralstonia sp. SJ98 toward co-metabolizable nitroaromatic compounds. Biochem Biophys Res Commun 2002; 299: 404-9. [ Links ]
11. Paul D, Singh R, Jain R. Chemotaxis of Ralstonia sp. SJ98 towards p-nitrophenol in soil. Environ Microbiol 2006; 8: 1797-804. [ Links ]
12. Tapilatu Y, Grossi V, Acquaviva M, Militon C, Bertrand J, Cuny P. Isolation of hydrocarbon-degrading extremely halophilic archaea from an uncontaminated hypersaline pond (Camargue, France). Extremophiles 2010; 14: 225-31. [ Links ]
13. Wolfe A, Berg H. Migration of bacteria in semisolid agar. Proc Natl Acad Sci USA 1989; 86: 6973-7. [ Links ]
14. Yang, Y.; Han, D. Feasibility of bioremediation for contaminated soil by halophilic bacteria. J Agro-Environ Sci, 2007;s1 [ Links ]
Recibido: 26/10/10
Aceptado: 22/02/11














