Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista argentina de microbiología
versión impresa ISSN 0325-7541
Rev. argent. microbiol. vol.43 no.3 Ciudad Autónoma de Buenos Aires jun./set. 2011
ARTÍCULO ORIGINAL
Antibiotic prescription in intensive care units in Latin America
Daniel J. Curcio on Behalf of the Latin American Antibiotic Use in Intensive Care Unit Group1
Correspondence. E-mail: djcurcio@gmail.com
ABSTRACT
The intensive care units (ICUs) are often considered as the epicenters of antibiotic resistance. Therefore, the total antibiotic consumption is approximately ten fold greater in ICU wards than in general hospital wards. The aim of this study was to evaluate the current use of antibiotics in Latin American ICUs. Three cross-sectional (one-day point) prevalence studies were undertaken in 43 Latin American ICUs. Of 1644 patients admitted, 688 received antibiotic treatment on the days of the study (41.8 %) and, 392 cases (57 %) were due to nosocomial-acquired infections. Of all infections, 22 % (151/688) corresponded to septic shock; and 22 % (151/688) to nosocomial pneumonia (50/151 [33 %], ventilator-associated pneumonia). In 485 patients (70.5 %), cultures were performed before starting antibiotic treatment. The most common microorganisms isolated were extended-spectrum ß-lactamase Enterobacteriaceae, (30.5 %), and Pseudomonas aeruginosa (17 %). Carbapenems (imipenem or meropenem) were the antibiotics most frequently prescribed (151/688, 22 %), followed by vancomycin (103/688, 15 %), piperacillin-tazobactam (86/688, 12.5 %) and broad-spectrum cephalosporins (mainly cefepime) (83/688, 12 %). In summary, carbapenems were the most frequent antibiotics prescribed in Latin American ICUs. This practice seems justified for the high rates of ESBL-producing Gram-negatives found in our patients. Beyond this reason, the problem of bacterial resistance in LA requires that physicians improve the use of carbapenems. The high prevalence of carbapenem-resistant A. baumannii and P. aeruginosa in the region, along with the prevalence of carbapenem-resistant Enterobacteriaceae, have increased markedly. A comprehensive evidence-based stewardship program based on local antimicrobial use and resistance problems should be implemented in our clinical settings.
Key words: Drug resistance; Carbapenem; ß-lactamases; Latin America.
RESUMEN
Prescripción de antibióticos en unidades de cuidados intensivos de Latinoamérica. Las unidades de cuidados intensivos (UCI) son a menudo consideradas el epicentro de la resistencia a los antibióticos. En este sentido, el consumo total de antibióticos es aproximadamente diez veces mayor en las UCI que en las salas de internación general. El objetivo de este estudio fue evaluar el hábito prescriptivo de antibióticos en las UCI de varios países de Latinoamérica. A tal fin, se realizó un estudio transversal con tres evaluaciones puntuales de un día de duración cada una, para determinar la prevalencia del uso de antibióticos en las 43 UCI ubicadas en distintos países del continente americano. De los 1644 pacientes admitidos, 688 estaban recibiendo tratamiento antibiótico en los días en que se realizó el relevamiento (41,8 %), en 392 casos (57 %), debido a infecciones nosocomiales. De todas las infecciones, 22 % (151/688) correspondieron a shock séptico y 22 % (151/688) a neumonía nosocomial (de estas últimas, el 33 % [50/151] fueron neumonías asociadas a ventilación mecánica). En 485 pacientes (70,5 %) se obtuvieron cultivos antes del inicio del tratamiento antibiótico. Entre los aislamientos, los microorganismos más comúnmente aislados fueron las enterobacterias productoras de ß-lactamasas de espectro extendido (BLEE) (30,5 %) y Pseudomonas aeruginosa (17 %). Los carbapenems (imipenem o meropenem) fueron los antibióticos prescriptos con mayor frecuencia (151/688, 22 %), seguidos por la vancomicina (103/688, 15 %), la piperacilina-tazobactama (86/688, 12,5 %) y las cefalosporinas de amplio espectro (principalmente cefepima) (83/688, 12 %). En conclusión, los carbapenems fueron los antibióticos más frecuentemente prescriptos en las UCI de los países latinoamericanos evaluados. Esta práctica podría estar justificada por las altas tasas de enterobacterias productoras de BLEE halladas en los pacientes de esas regiones. Más allá de esta razón, el problema de la resistencia bacteriana en muchos países del continente requiere que los médicos optimicen el uso de los carbapenems, ya que la prevalencia de aislamientos resistentes a este grupo de antimicrobianos se ha incrementado marcadamente, tanto en A. baumannii y P. aeruginosa como en enterobacterias. Frente a este panorama, en todos estos países se torna necesario implementar programas de optimización del uso de antibióticos, basados en la epidemiología y en las tasas de resistencia locales.
Palabras clave: Resistencia a los antibióticos; Carbapenem; ß-lactamasas; Latinoamérica.
INTRODUCTION
Antibiotics are one of the most common therapies administered in intensive care unit (ICUs) settings (9). Hospitalized patients in these areas often suffer from a debilitated physical condition and deficiencies of the immune system, requiring intense antibiotic therapy for longer periods and for severe infectious complications, including nosocomial infections. Therefore, the total antibiotic consumption is approximately tenfold greater in ICUs than in general hospital wards (44).
This high density of antibiotic use in the ICUs favours the development of multi-drug resistant pathogens (MDR), either by selecting a resistant mutant or allowing the emergence of MDR-bacteria in the colonisation flora (14, 21, 34, 45). Rice recently referred to these as the "ESKAPE" pathogens (42) (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) to emphasize that they currently cause the majority of world-wide hospital infections (Latin America included) (9, 50), and effectively "escape" the effects of antibacterial drugs.
Taking these data into account, the aim of this study was to evaluate the current use of antibiotics in Latin American ICUs.
MATERIAL AND METHODS
Three cross-sectional (one-day point) prevalence studies have been performed on November 17, 2008; June 11, 2009, and September 23, 2010 in 68 LA ICUs in order to gather information about antibiotic prescription.
All participant LA ICUs completed a web-based data collection form with data from the patients receiving antibiotics, with the exception of those who received them for surgical prophylaxis. The data was collected using a unique electronic form included in the website ATB-Terapia IntensivaTM (http://www.clinicalrec.com.ar), designed by ClinicalREC® from Argentina.
The participating hospitals were from Argentina (n = 9), Bolivia (n = 7), Chile (n = 8), Colombia (n = 16), Costa Rica (n = 2), Ecuador (n = 16), Guatemala (n = 1), Peru (n = 5) and Venezuela (n = 4).
Each ICU had a principal investigator, who was given a personal username and password to access the electronic form. All of them received training in the method to use the website.
The following data was only recorded for patients with antibiotic treatment in the ICU (prophylaxis was not included):
- General data of the ICU: number of beds; number of patients admitted; number of patients admitted with antibiotic treatment.
- General data of the patients: number of registry, sex, age, date of ICU admission, risk factors for infection due to MDR pathogens, and severity of illness at admission (measured by the APACHE II score).
- Infection data: date of diagnosis of infection, source of infection (community or nosocomial-acquired), diagnosis when the antibiotic treatment was started, and microbiological documentation.
- Antibiotic use data: severity of the disease at the beginning of the antibiotic treatment (measured by the SOFA score), type of indication (i-empirical treatment -patient with signs and symptoms of infection and pending cultures-, ii-culture-directed therapy -patient with signs and symptoms of infection and positive cultures-or iii-clinically documented infection -patient with signs and symptoms of infection without cultures or with negative cultures); previous antibiotic therapy during the present hospitalization (type and days of antibiotics used); and antibiotic treatment of the present infection (type and days of antibiotics used).
Established criteria were used to define clinical infection (15). An infection was considered nosocomial when it was not present or incubating in a patient at the time of admission to a hospital, but occurred within >48 h after admission to the hospital (15, 19). Infections occurring within 48 h of admission to the hospital were considered community-acquired, unless the patients had been transferred directly from another hospital or nursing home or discharged from a hospital within 30 days preceding admission to the hospital (19).
Bacterial identification was performed according to the clinical microbiology procedure handbook (26). Bacterial identification was confirmed, and antibiotic susceptibility testing was performed on each of the isolates using a semi-automated system in 30 hospitals (59 %). In the remaining hospitals, bacterial susceptibility was determined by using the Kirby Bauer method (disk diffusion).
Extended-spectrum ß-lactamase (ESBL)-producing microorganisms were detected and confirmed according to the standards of the Clinical Laboratory Standards Institute, using the double disk test for confirmation (8).
For the analysis, carbapenems, vancomycin, piperacillin-tazobactam, broad-spectrum cephalosporins, tigecycline, polymixins and linezolid were considered as "restricted antibiotics" based on their epidemiological and economic implications in the hospitals.
The study was directed in compliance with the clinical routine practices determined by the responsible physician. The study was based on a case registry methodology and did neither require the prescription of specific drugs or other treatments nor the performance of procedures or diagnostic tests other than those prescribed by the responsible physician. The study was approved by the local institutional review board of each participant's hospitals and patient consent was not required because of the observational nature of the study.
Results are expressed as proportions. When applicable, two tailed hypothesis testing for difference in proportions were used (Proportion Test); a p value of < 0.05 was considered significant.
RESULTS
Patients' general data
On the three days of the prevalence study, there were 1644 patients in the participating ICUs (1927 total beds), 688 of whom (41.8 %, range between ICUs 0-100 %) were receiving antibiotics.
Median age of patient was 60.3 years (range 14-93); 471 were male (63 %).
Median of APACHE II score at admission was 18 (SD ± 5) (≥ 15 in 482/688 patients -70 %-, and < 15 in 206/688 patients -30 %-) (Table 1).
Table 1. General and infection data of patients
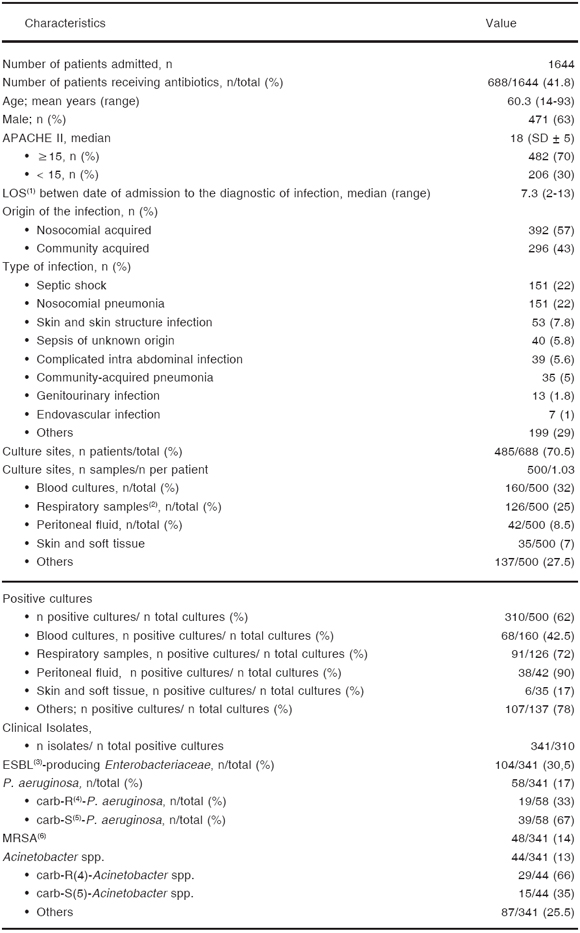
(1)length of stay; (2)traqueal aspirate (25%), bronchoalveolar lavage -BAL-(1.5%), and mini-BAL (2%); (3)extended-spectrum ß-lactamases (K. pneumoniae and Escherichia coli); (4)carbapenem-resistant; (5)carbapenem-susceptible; (6)methicillin-resistant S. aureus
The prevalence of risk factors for infections due to MDR pathogens was 100 % in this patient population. Hospitalization in an acute care hospital for 2 or more days within the past 90 days, recipients of recent intravenous antibiotic therapy, and immunosuppressive illness or therapy were the risk factors more frequently observed (69 %, 51 % and 30 %, respectively) (Table 2).
Infection data
The mean of length of stay (LOS) in an ICU from admission date to infection diagnosis was 7.3 days (range 2-13 days).
The indication for antibiotic treatment corresponded to community-acquired infections in 43 % (n = 296) and to nosocomial-acquired infections in 57 % (n = 392) of cases.
The most frequent infections were: septic shock (22 % [151/688]), nosocomial pneumonia (22 % [151/688]), complicated skin and skin structure infections (7.8 % [53/688]), sepsis of unknown origin (5.8 % [40/688]), and complicated intrabdominal infections (5.6 % [39/688]) (Table 1). Thirty-three percent of nosocomial pneumonia were ventilator-associated (VAP) (50/151).
Five hundred samples for bacterial culture were obtained before starting antibiotic therapy in 485 patients (70.5 %). Thirty-two percent of the samples were from blood (160/485), whereas 25 % (126/500) were from the respiratory tract (tracheal aspirate -23 %-, bronchoalveolar lavage [BAL] -1 %-, and mini-BAL 1 %).
In 62 % of the cultures performed (310/500), a microorganism considered as the causative agent of the infection was isolated (72 % in respiratory tract samples and in 42.5 % of blood cultures) (Table 1).
Among isolates, extended-spectrum ß-lactamase Enterobacteriaceae, -mainly K. pneumoniae and E. coli- (30.5 %), and P. aeruginosa (17 %) were the most common microorganisms, followed by methicillin-resistant S. aureus -MRSA- (14 %), and Acinetobacter spp. (13 %), 66 % of Acinetobacter spp. and 33 % of P. aeruginosa, were carbapenem-resistant -imipenem and meropenem- respectively (Table 1).
Antibiotic use data
Median of SOFA score at the beginning of the antibiotic treatment was 7 (SD ± 4.2) (≥ 6 in 564/688 patients-82 %-, and < 6 in 124/688 patients -18 %-) (Table 3).
Table 3. Patient antimicrobial prescription data (n = 688)
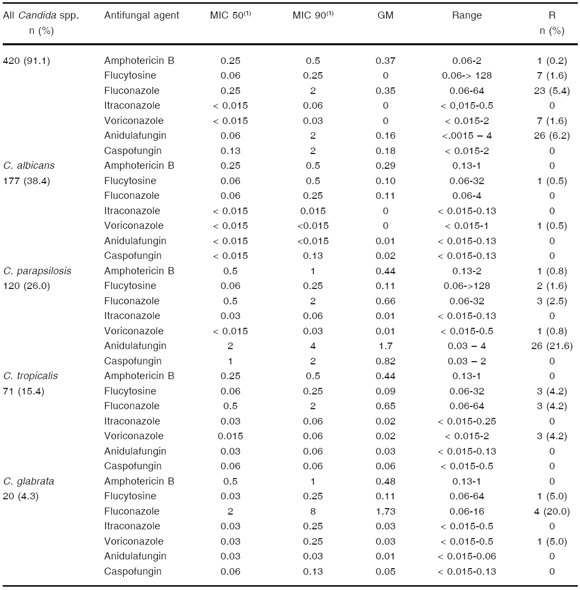
(1)alone or in combination with other antibiotics
We found that antimicrobial therapy was prescribed on the study day as empirical treatment, culture-directed prescription and clinically documented infection in 52 %, 32 %, and 12 %, respectively. At no point were antibiotics discontinued, not even in cases in which cultures were not done or yielded negative results (Table 3).
Fifty-eight percent of the patients (399/688) had received previous antibiotic therapy during current hospitalization, (≥ 3 days of treatment in 320/399 patients [80.2 %], and < 3 days of treatment in 79/399 patients [19.8 %]). Third generation cephalosporins, fluoroquinolones, carbapenems and vancomycin (in all cases alone or in combination with other antibiotics) were the most frequent antibiotics previously used (42 %, 24 %, 24 % and 23 %, respectively) (Table 3).
On the days of the study, carbapenems (imipenem or meropenem) were the most frequently prescribed antibiotics (151/688, 22 %), followed by vancomycin (103/688, 15 %) and piperacillin-tazobactam (86/688, 12.5 %) and broad-spectrum cephalosporins (mainly cefepime) (83/688, 12 %) (Table 3). Carbapenems were prescribed in combination with vancomycin in 67 % (101/151) of the cases.
There were no significant differences in "restricted" antibiotic prescriptions (carbapenems, vancomycin, piperacillin-tazobactam, broad-spectrum cephalosporins, tigecycline, polymixins and linezolid) among the four groups of APACHE II and SOFA score at the beginning of the antibiotic treatment (< 15 and ≥ 15 and < 6 and ≥ 6, respectively) (Table 4).
Table 4. Prescription of "restricted" antibiotics in patients with different severity scores values
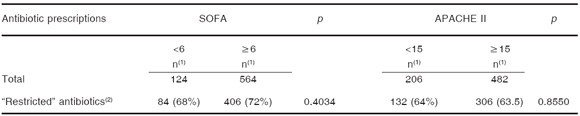
(1)number of prescriptions, (2)carbapenems, vancomycin, piperacillin-tazobactam, broad-spectrum cephalosporins, tigecycline, polymixins and linezolid
DISCUSSION
The results of this observational, cross-sectional study shows that 41.8 % of patients hospitalized in a general LA ICU were receiving at least one antibiotic, in 57 % of the cases to treat nosocomial infections. Previously published studies show a wide range of prevalence of antibiotic use in the ICU setting (between 45-85 %) (5, 16, 40, 54). There are several reasons for the high consumption of antibiotics in the ICU (5, 16, 36, 40, 54): i-patients admitted with serious community-acquired infections (ie. community-acquired pneumonia and complicated intra abdominal infection), and ii-the acquisition of the infection during the nosocomial stay, favored by the presence of multiple comorbidities, the high rates of invasive device use and the presence of risk factors for infections due to MDR pathogens (17, 36, 37). In that sense, all our patients presented at least one risk factor for infections due to MDR bacteria, mainly hospitalization for 2 or more days and previous antibiotic treatment within the past 90 days in both cases.
In order to improve these antibiotic prescription patterns, it is well-established that the precise knowledge of the pathogens associated with the disease allows a rational antibiotic selection. In clinical practice, however, antibiotics are often used even when culture results are not available (10). It is the rational of the de-escalation strategy which proposes to reassess the patient on day 3 to define the initial antibiotic treatment based on culture results and the clinical response (23, 24). In our patients, cultures were obtained before beginning the antibiotic treatment in 70.5 % of the cases. In more than 60 % of these patients, a microorganism considered as the causative agent of the infection was isolated; nevertheless, only 32 % of patients received a culture-directed antibiotic prescription. In a large European study which included 20 hospitals, Ansari et al. (2) have recently published that samples for bacterial culture were obtained before therapy only in 43 % of cases (1822 of 4242 adult patients).
As demonstrated by Erlandsson et al. (11), septic shock and nosocomial pneumonia account for nearly one half of all antibiotics prescribed to our patients. In these particular indications, it is well-established that the appropriate empirical antimicrobial treatment is associated with better survival, therefore several authors recommend the use of broad spectrum antibiotics (alone or in combination) for the empirical treatment of these serious infections (20, 31, 41). However, not to consider the tailored therapy in these cases could lead to the possibility of "collateral damage", where overuse/misuse of antibiotics is associated with MDR-pathogen infections (43). The low level of intent to document the microbiology of the infections (especially in severely ill patients) increases this possibility. The challenge for ICU physicians is to understand that obtaining microbiological cultures before initiating empirical antimicrobial therapy is part of the diagnostic work-up of ICU patients (49).
"ESKAPE pathogens" (with the exception of E. faecium) were the most common microorganisms isolated from our patients (74.5 %), with a similar MDR-profile to those described by several microbiological surveillance systems in the region (47, 50). The TEST program (Tigecycline Evaluation and Surveillance Trial), has found that rates of ESBL-K.pneumoniae, and carbapenem-resistant A. baumannii and P. aeruginosa were higher in Latin America than in North America and Europe (37.9 %; 37.6 %; 35.8 % vs. 9.7 %; 13.1 %, 15.1 % and 15.3 %; 14.8 %; 17.4 %, respectively). In contrast, rates of methicillin resistance among S. aureus were higher in North America (53.7 %) than in Latin America (46.6 %) and Europe (25.1 %) (50).
We found that the prevalence of MRSA and Acinetobacter spp. were lower than other series published by LA authors (1, 3, 4). The low frequency of VAP described in our patients (7.2 %, 50/688), where these microorganisms are the most frequent etiologic agents in our region, could be the reason of this finding.
Prior antibiotic usage is one factor that predisposes to infections with MDR-bacteria (6, 7, 12). In 58 % (80.2 % ≥ 3 days) of the cases in our study, the evaluated patients had received previous antibiotic treatment, most of them broad-spectrum agents). In one third of the patients, broad-spectrum cephalosporins or fluoroquinolones were used, both of which are in close relationship with the selection of MDR microorganisms, mainly ESBL-producing gram-negatives, the microorganisms most frequently isolated in our study (27, 32, 48).
In terms of antibiotic prescription on the day of the study, we observed that carbapenems (imipenem or meropenem, alone or in combination with vancomycin), were the most frequently prescribed antibiotics in Latin American ICUs, followed by vancomycin, piperacillin-tazobactam and broad-spectrum cephalosporins.
This pattern of prescription habits seems justified for several reasons: i-sixty-four percent of the registered infections were nosocomial, where MDR-microorganisms are frequently involved (13); ii-fifty-eight percent of patients had received previous antibiotic therapy other than carbapenems during the present hospitalization in more than prescribed antibiotic which is a very common concept between ICU-physicians; iii-high rates of ESBL-producing gram-negatives were found in our patients. Carbapenems are stable against hydrolyzing activity of ESBLs and are regarded as the drugs of choice for the treatment of infections caused by ESBL-producing Enterobacteriaceae. The combination with vancomycin extends the spectrum towards MRSA; iv-the early effective therapy for infections in critically ill patients (defined as antimicrobial treatment against pathogens associated with infection) is associated with low mortality rates (20, 22, 25); therefore, a fresh approach to the effective treatment of septic shock and nosocomial pneumonia (the most frequent pathologies in our study), is to use a broad-spectrum antibacterial treatment followed by precision therapy based on susceptibility results (41), and v-physicians believe in carbapenems because it is a potent antibiotic having an ultra-broad spectrum of activity that encompasses MDR and difficult-to-treat gram-negative bacteria with several clinical trial data that support its clinical effectiveness. In fact, we have found that there is a trend to use carbapenems in severely ill patients (alone or in combination with vancomycin), regardless of the microbiological documentation.
Beyond these reasons, there are several issues related with the antibiotics most frequently used in Latin American ICUs (carbapenems and vancomycin), which physicians must take into account.
The high prevalence of carbapenem-resistant A. baumannii in the region has increased markedly (51), along with the prevalence of carbapenem-resistant strains of P. aeruginosa (47). Another concern is the description in LA of Enterobacteriaceae isolates (particularly K. pneumoniae) that possess carbapenem-hydrolyzing enzymes belonging to the KPC family of ß-lactamases [Colombia (52, 53), Brazil (35, 39), and Argentina (38)]. In fact, the increased use of carbapenems to combat the growing prevalence of MRB, particularly ESBL-producing strains, shows early signs of eroding carbapenem effectiveness. A more highly targeted and restrained use of these drugs, aimed at preserving their antimicrobial activity, is probably warranted. Their therapeutic substitution in specific pathologies is one of the strategies to reach this objective; for example, the use of tigecycline instead of carbapenems in intra abdominal infections where ESBL-producing gram-negatives are suspected.
The vancomycin MIC creep in MRSA is a worldwide problem which limits the use of this antibiotic in severely ill patients. In respiratory infections, such as VAP or bacteremia due to MRSA, the clinical success using vancomycin was associated with the possibility of reaching a pharmacodynamic target of > 350 of AUC/MIC (18). When the MRSA has a vancomycin MIC ≥ 1mg/ml (more than 70 % of the LA isolates) (18), the probability of achieving this AUC/MIC target with the standard dose is between 40 % and 60 %, and when the MRSA vancomycin MIC these cases we need higher doses of vancomycin, with the consequent risk of nephrotoxicity (28, 30). Therapeutic options other than vancomycin should be considered in patients with risk factors for infection due to MRSA with high vancomycin MIC (ie. patients' recent exposure to vancomycin) (29).
We did not find significant differences between antibiotic prescription (global and "restricted" antibiotics), in different groups of patients based on the mean of the APACHE II score at admission (< 15 and ≥ 15) and the SOFA score at the beginning of the antibiotic treatment (< 6 and ≥6). Our observation revealed that the main condition to select the antibiotic treatment in our hospitals was the fact of "being a patient in ICU" rather than the severity of illness. Whether this is due to a low potential of our observational methodology or the small number of cases analyzed needs further evaluation.
Our findings show that our web-based method for collection of one-day point prevalence was implemented successfully. However, based on the limitations of the model used, the results of this study must be taken with caution.
We hope that the limitations of our current study may generate enthusiasm for prospective studies having more robust designs, in order to improve our knowledge on antibiotic prescription habits in Latin American ICUs.
1Latin American Antibiotic Use in Intensive Care Unit Group: Daniel Curcio, Instituto Sacre Coeur, Argentina; Abraham Alí, Fundación Cardioinfantil, Colombia; Alejandro Duarte, Hospital Regional Río Grande, Argentina; Alfredo Defilippi Pauta, Hospital Luis Vernaza, Ecuador; Alvaro Fernando Erazo Guerrón, Hospital Quito Nro 1 Policía Nacional, Ecuador; Ana Bañales Churrut, Hospital San José, Chile; Blanca Salcedo, Hospital Oncológico Solón Espinosa Ayala, Ecuador; Carlos Humberto Paz Chávez, Hospital Central de la Fuerza Aérea y Clinica San Gabriel, Perú; Carlos Ibáñez-Guzmán, Hospital Obrero N° 1, Bolivia; Carlos Rebolledo Maldonado, Clínicas SaludCoop, Colombia; Daniel Alvarado Cueto, Clínica Madre Bernarda, Colombia; David López García, Hospital Universitario San Vicente de Paúl, Colombia; David Yepes, Clinica CES, Colombia; Eduardo Labarca, Hospital Naval Almte Nef, Chile; Electra Moreno Veloz, Hospital de Infectología Dr. Daniel Rodriguez Maridueña, Ecuador; Erick Valencia, Clínicas Saludcoop, Colombia; Fabian Plano, Hospital Privado Modelo, Argentina; Fabio Varón, Fundación Cardioinfantil, Colombia; Fausto Guerrero Toapanta, Hospital Carlos Andrade Marin, Ecuador; Fernando Paredes Oña, Hospital de Clínicas Pichincha, Ecuador; Francisco Arancibia, Instituto Nacional del Tórax, Chile; Francisco Molina Saldarriaga, Clínica Universitaria Bolivariana, Colombia; Franco Montufar Andrade, Hospital Pablo Tobón Uribe, Colombia; Freddy Morales Alava, Hospital Oncológico Dr. Julio Villacreses Colmont, Ecuador; Gleinner Cañarte Bermudez, Hospital IEES de Portoviejo, Ecuador; Gustavo La Fuente Zerain, Hospital Universitario Japonés, Bolivia; Henry Caballero Narváez, Hospital Enrique Garcés, Ecuador; Iván Ramos Palomino, Clínica San Gabriel, Perú; Juan Salas Villasante, Hospital Regional Docente de Trujillo, Perú; Juan Villalobos Vindas, Hospital México, Costa Rica; Jorge Castagnino, Sanatorio de La Providencia, Argentina; Jorge Espitaleta Gómez, Hospital San Rafael de Alajuela, Costa Rica; Jorge Ranero Meneses, Instituto Guatemalteco de Seguridad Social, Guatemala; José Rojas Suarez, Gestión Salud SA and Grupo de Investigación en Cuidados Intensivos y Obstetricia, Colombia; Juan Carlos Fernández Mercado, Gestión Salud SA and Grupo de Investigación en Cuidados Intensivos y Obstetricia, Colombia; José Guzmán Torrico, Centro Médico Quirúrgico Boliviano Belga, Bolivia; José Vergara Centeno, Hospital Luis Vernaza, Ecuador; Juan Carlos Medina, Sanatorio Itoiz, Argentina; Julio Durán Pérez, Hospital Uninorte, Colombia; Karina Marín, Hospital Oncológico Solón Espinosa Ayala, Ecuador; Lenny Alba Caero, Hospital Clínico Viedma, Bolivia; Leonardo Durán Crespo, Hospital Universitario Univalle, Bolivia; Leonina Ferreira Cabrera, Hospital Guillermo Grant Benavente, Chile; Luis Castillo Bravo, Instituto Enfermedades Neoplasicas, Perú; Luis Soto Germani, Hospital San Pablo De Coquimbo, Chile; Martín Carvajal Herrera, Clínica Medihelp Services, Colombia; Martin Oyanguren, Hospital Nacional Edgardo Rebagliati, Perú; Miguel Chung Sang, Clinica Guayaquil and Hospital Militar de Guayaquil, Ecuador; Miguel Llano, Hospital General de las Fuerzas Armadas, Ecuador; Mijail Játiva, Hospital Eugenio Espejo, Ecuador; Norma Ruiz Oliveros, Hospital Militar de Caracas, Venezuela; Oscar López Acosta, Clínica Martha, Colombia; Rafael Cotes, Clínica Sur, Colombia; Rafael Thomen, Clínica La Asunción, Colombia; Ray Mendoza Franco, Hospital Consorcio Gestión UCI, Colombia; Ramón Belloni, Sanatorio Güemes, Argentina; Ricardo Morales, Clínica Las Lilas, Chile; Ricardo Romero, Clínica Sagrada Familia and Clínica Adventista Belgrano, Argentina; Rolando Aguilera Perrogón, Hospital Obrero Nro 3, Bolivia; Ruben Camacho Alarcón, Clínica San Gregorio and Hospital IESS-Manta, Ecuador; Ruben Camargo, Clinica General del Norte, Colombia; Stenio Cevallos, Hospital Alcivar, Ecuador; Stevens Salva Sutherland, Hospital de Clinicas Caracas, Venezuela; Victor Alanis Mirones, Hospital Universitario San Juan de Dios, Bolivia; Víctor Figueroa, Hospital Oncológico Solón Espinosa Ayala, Ecuador; Virginia Intriago Cedeño, Hospital Verdi Cevallos Balda, Ecuador; Viviana Romero, Hospital Padre Pedro Tardivo, Argentina; Werner Jensen, Hospital Dr. Gustavo Fricke, Chile; Zulma Urbina Contreras, Clínica Universitaria del Norte de Santander, Colombia; Alfonso Socorro Morales, Hospital Dr. Manuel Noriega Trigo, Venezuela; Arnoldo Rivero Mendoza, Hospital Dr. Miguel Oraa, Venezuela; Vinko Tomicic Flores, Clínica Alemana, Chile.
Acknowledgements. Argentina: Juan Herrera Paz (Instituto Sacre Coeur); Gerardo Filippa and Eduardo Perrault (Hospital Regional Río Grande); Gonzalo Cortés y Tristán (Sanatorio Itoiz); Raul Zambón (Hospital Privado Modelo); Ivan Vite Acosta (Sanatorio San José); Bolivia: Kathia Laguna, Sheilla Tellez, Karen Torrez, Richard Orellana and Sergio Peca (Hospital Universitario Japonés); Carlos Alfaro Claros (Hospital Clínico Viedma); Ariel Quina Jimenez (Hospital Universitario Univalle); Erika Dájer Ulloa (Hospital Obrero Nro 3); Chile : Angel Riveron Soler (Hospital San José); Javiera Gajardo Arias (Hospital San Pablo De Coquimbo); Colombia: Fabio Barón (Fundación Cardioinfantil); Luis Carlos Julio Narváez (Clínica Madre Bernarda); Argemiro de J. Gallego Ossa, Jorge Andrés Ochoa Vásquez and Luis Horacio Atehortúa López (Hospital Universitario San Vicente de Paúl); Jezid Miranda Quintero (Gestión Salud SA and Grupo de Investigación en Cuidados Intensivos y Obstetricia), Rubén Teherán (Hospital Consorcio Gestión UCI), Ana Lucia Rangel Colmenares and Ernesto Alberto Mendoza (Clínica Universitaria del Norte de Santander); Ana Lucia Rangel Colmenares, Ernesto Alberto Mendoza Hernández, Rafael Olarte Ardila (Clínica Universitaria del Norte de Santander), Miguel Coral Pabón (Instituto del Corazón Ibagué and IPS Clínica Caprecom Ibagué, Colombia), César Enciso Olivera (Hospital Infantil Universitario de San José, Colombia); Mario Villabón Gonzalez and Camilo Velez (Hospital San José, Colombia); Ecuador: Guillermo Falconi (Hospital Carlos Andrade Marin); Luis González and Gonzalo Sánchez Sánchez (Hospital Luis Vernaza); José Guerrero Carrión (Hospital General de las Fuerzas Armadas Nro 1); Bosco Fabian Mendoza Cedeño, Johanna Macías, Blanchy Macías Romero, Otto Intriago Montesdeoca and Guido Macias García (Hospital Oncológico Dr. Julio Villacreses Colmont); Karina Marín (Hospital Oncológico Solón Espinosa Ayala); Alvaro Villacrés, Juan Carlos Carrión and Emérita Basantes (Hospital Oncológico Solón Espinosa Ayala); Enrique Boloña (Clínica Guayaquil); Killen Briones Claudett (Hospital Militar de Guayaquil); Gustavo Granda (Hospital Eugenio Espejo); Jorge Vera (Hospital Verdi Cevallos Balda); El Salvador: Gerardo Campos Mezquita and Corina Esmeralda Arevalo Huezo (Hospital General. ISSS); Guatemala: Rudy López (Instituto Guatemalteco de Seguridad Social); Perú: Deysi Diaz Seijas (Clínica San Gabriel).
1. Alvarez C, Cortes J, Arango A, Correa C, Leal A; Grupo para el Control de la Resistencia Bacteriana en Bogotá. Antimicrobial resistance in intensive care units in Bogotá, Colombia, 2001-2003. Rev Salud Publica (Bogotá) 2006; Suppl 1: 86-101. [ Links ]
2. Ansari F, Erntell M, Goossens H, Davey P. The European surveillance of antimicrobial consumption (ESAC) point-prevalence survey of antibacterial use in 20 European hospitals in 2006. Clin Infect Dis 2009; 49: 1496-504. [ Links ]
3. Bantar C, Famiglietti A, Goldberg M. Three-year surveillance study of nosocomial bacterial resistance in Argentina. The Antimicrobial Committee; and the National Surveillance Program (SIR) Participants Group. Int J Infect Dis 2000; 4: 85-90. [ Links ]
4. Bantar C, Famiglietti A, Radice M, Quinteros M, Antimicrobial Committee; SIR Participants Group. Participants Group. A 7-year national survey on bacterial resistance in bronchoalveolar lavage from patients hospitalized in Argentina. Diagn Microbiol Infect Dis 2008; 60: 65-9. [ Links ]
5. Bergmans DC, Bonten MJ, Gaillard CA, van Tiel FH, van der Geest S, de Leeuw PW, Stobberingh EE. Indications for antibiotic use in ICU patients: a one-year prospective surveillance. J Antimicrob Chemother 1997; 39: 527-35. [ Links ]
6. Celis R, Torres A, Gatell JM, Almela M, Rodriguez-Roisin R, Agusti-Vidal A. Nosocomial pneumonia. A multivariate analysis of risk and prognosis. Chest 1988; 93: 318-24. [ Links ]
7. Chow JW, Fine MJ, Shlaes DM, Quinn JP, Hooper DC, Johnson MP, Ramphal R, Wagener MM, Miyashiro DK, Yu VL. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med 1991; 115: 585-90. [ Links ]
8. Clinical and Laboratory Standard Institute. Clinical and Laboratory Standard Methods. Performance Standards for Antimicrobial Susceptibility Testing, 17th Informational Supplement; 2007; M100-S17. Wayne, PA. EE.UU. [ Links ]
9. Curcio D, Alí A, Duarte A, Defilippi Pauta A, Ibáñez-Guzmán C, Chung Sang M, Latin American Antibiotic Use in Intensive Care Unit Group. Prescription of antibiotics in intensive care units in Latin America: an Observational Study. J Chemother 2009; 21: 41-8. [ Links ]
10. Cuthbertson BH, Thompson M, Sherry A, Wright MM, Bellingan GJ; Intensive Care Society. Antibiotic-treated infections in intensive care patients in the UK. Anaesthesia 2004; 59: 885-90. [ Links ]
11. Erlandsson M, Burman LG, Cars O, Gill H, Nilsson LE, Walther SM, Hanberger H; Strama-Icu Study Group. Prescription of antibiotic agents in Swedish intensive care units is empiric and precise. Scand J Infect Dis 2007; 39: 63-9. [ Links ]
12. Fagon JY, Chastre J, Domart Y, Trouillet JL, Pierre J, Darne C, Gibert C. Nosocomial pneumonia in patients receiving continuous mechanical ventilation. Prospective analysis of 52 episodes with use of a protected specimen brush and quantitative culture techniques. Am Rev Respir Dis 1989; 139: 877-84. [ Links ]
13. Fridkin SK, Steward CD, Edwards JR, Pryor ER, McGowan JE Jr, Archibald LK, Gaynes RP, Tenover FC. Surveillance States hospitals: project ICARE phase 2. Project Intensive Care Antimicrobial Resistance Epidemiology (ICARE) hospitals. Clin Infect Dis 1999; 29: 245-52. [ Links ]
14. Fujimura S, Nakano Y, Sato T, Shirahata K, Watanabe A. Relationship between the usage of carbapenem antibiotics and the incidence of imipenem-resistant Pseudomonas aeruginosa. J Infect Chemother 2007; 13: 147-50. [ Links ]
15. Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control 1998; 16: 128-40. [ Links ]
16. Hanssens Y, Ismaeil BB, Kamha AA, Elshafie SS, Adheir FS, Saleh TM, Deleu D. Antibiotic prescribing pattern in a medical intensive care unit in Qatar. Saudi Med J 2005; 26: 1269-76. [ Links ]
17. Harris AD, McGregor JC, Johnson JA, Strauss SM, Moore AC, Standiford HC, Hebden JN, Morris JG Jr. Risk factors for colonization with extended-spectrum beta-lactamase-producing bacteria and intensive care unit admission. Emerg Infect Dis 2007; 13: 1144-9. [ Links ]
18. Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med 2006; 166: 2138-44. [ Links ]
19. Hyle EP, Lipworth AD, Zaoutis TE, Nachamkin I, Bilker WB, Lautenbach E. Impact of inadequate initial antimicrobial therapy on mortality in infections due to extended-spectrum beta-lactamase-producing Enterobacteriaceae: variability by site of infection. Arch Intern Med 2005; 165: 1375-80. [ Links ]
20. Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloods-tream infections on patient outcomes in the ICU setting. Chest 2000; 118: 146-55. [ Links ]
21. Iosifidis E, Antachopoulos C, Tsivitanidou M, Katragkou A, Farmaki E, Tsiakou M, Kyriazi T, Sofianou D, Roilides E. Differential correlation between rates of antimicrobial drug consumption and prevalence of antimicrobial resistance in a tertiary care hospital in Greece. Infect Control Hosp Epidemiol 2008; 29: 615-22. [ Links ]
22. Kollef MH. Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients. Clin Infect Dis 2000; Suppl 4: S131-S138. [ Links ]
23. Kollef MH. Hospital-acquired pneumonia and de-escalation of antimicrobial treatment. Crit Care Med 2001; 29: 1473-5. [ Links ]
24. Kollef MH. Providing appropriate antimicrobial therapy in the intensive care unit: surveillance vs. de-escalation. Crit Care Med 2006; 34: 903-5. [ Links ]
25. Kollef MH, Sherman G, Ward S, Fraser VJ. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 1999; 115: 462-74. [ Links ]
26. Krieg NR, Holt JG. Bergey´s Manual of Systematic Bacteriology, Volume 1. Baltimore, MD, USA: Williams & Wilkins, 1984. [ Links ]
27. Landman D, Quale JM, Mayorga D, Adedeji A, Vangala K, Ravishankar J, Flores C, Brooks S. Citywide clonal outbreak of multiresistant Acinetobacter baumannii and Pseudomonas aeruginosa in Brooklyn, NY: the preantibiotic era has returned. Arch Intern Med 2002; 162: 1515-20. [ Links ]
28. Lodise TP, Lomaestro B, Graves J, Drusano GL Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother 2008; 52: 1330-6. [ Links ]
29. Lodise TP, Miller CD, Graves J, Evans A, Graffunder E, Helmecke M, Stellrecht K. Predictors of high vancomycin MIC values among patients with methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother 2008; 62: 1138-41. [ Links ]
30. Lodise TP, Graves J, Evans A, Graffunder E, Helmecke M, Lomaestro BM, Stellrecht K. Relationship between vancomy Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob Agents Chemother 2008; 52: 3315-20. [ Links ]
31. Luna CM, Vujacich P, Niederman MS, Vay C, Gherardi C, Matera J, Jolly EC. Impact of BAL data on the therapy and outcome of ventilator-associated pneumonia. Chest 1997; 111: 676-85. [ Links ]
32. Mentzelopoulos SD, Pratikaki M, Platsouka E, Kraniotaki H, Zervakis D, Koutsoukou A, Nanas S, Paniara O, Roussos C, Giamarellos-Bourboulis E, Routsi C, Zakynthinos SG. Prolonged use of carbapenems and colistin predisposes to ventilator-associated pneumonia by pandrug-resistant Pseudomonas aeruginosa. Intensive Care Med 2007; 33:1524-32. [ Links ]
33. Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet 2004; 43: 925-42. [ Links ]
34. Monnet DL, Archibald LK, Phillips L, Tenover FC, McGowan JE Jr, Gaynes RP. Antimicrobial use and resistance in eight US hospitals: complexities of analysis and modeling. Intensive Care Antimicrobial Resistance Epidemiology Project and National Nosocomial Infections Surveillance System Hospitals. Infect Control Hosp Epidemiol.1998; 19: 388-94. [ Links ]
35. Monteiro J, Santos AF, Asensi MD, Peirano G, Gales AC. First report of KPC-2-producing Klebsiella pneumoniae strains in Brazil. Antimicrob Agents Chemother 2009; 53: 333-4. [ Links ]
36. Nguyen HB, Rivers EP, Havstad S, Knoblich B, Ressler JA, Muzzin AM, Tomlanovich MC. Critical care in the emergency department: A physiologic assessment and outcome evaluation. Acad Emerg Med 2000; 7: 1354-61. [ Links ]
37. Parker CM, Kutsogiannis J, Muscedere J, Cook D, Dodek P, Day AG, Heyland DK; Canadian Critical Care Trials Group. Ventilator-associated pneumonia caused by multidrug-resistant organisms or Pseudomonas aeruginosa: prevalence, incidence, risk factors, and outcomes. J Crit Care 2008; 23: 18-26. [ Links ]
38. Pasteran FG, Otaegui L, Guerriero L, Radice G, Maggiora R, Rapoport M, Faccone D, Di Martino A, Galas M. Klebsiella pneumoniae carbapenemase-2, Buenos Aires, Argentina. Emerg Infect Dis 2008; 14: 1178-80. [ Links ]
39. Peirano G, Seki LM, Val Passos VL, Pinto MC, Guerra LR, Asensi MD. Carbapenem-hydrolysing beta-lactamase KPC-2 in Klebsiella pneumoniae isolated in Rio de Janeiro, Brazil. J Antimicrob Chemother 2009; 63: 265-8. [ Links ]
40. Petersen IS, Hesselbjerg L, Jørgensen L, Renstrup J, Barnung S, Schierbeck J, Jepsen OB. High antibiotic consumption in Danish intensive care units? APMIS 1999; 107: 989-96. [ Links ]
41. Rello J, Gallego M, Mariscal D, Sonora R, Valles J. The value of routine microbial investigation in ventilator-associated pneumonia. Am J Respir Crit Care Med 1997; 156: 196-200. [ Links ]
42. Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis 2008; 197: 1079-81. [ Links ]
43. Richards GA. The therapeutic challenge of Gram-negative sepsis: prolonging the lifespan of a scarce resource. Clin Microbiol Infect 2005; Suppl 6: 18-22. [ Links ]
44. Røder BL, Nielsen SL, Magnussen P, Engquist A, Frimodt-Møller N. Antibiotic usage in an intensive care unit in a Danish university hospital. J Antimicrob Chemother 1993; 32: 633-42. [ Links ]
45. Rogues AM, Dumartin C, Amadéo B, Venier AG, Marty N, Parneix P, Gachie JP. Relationship between rates of antimicrobial consumption and the incidence of antimicrobial resistance in Staphylococcus aureus and Pseudomonas aeruginosa isolates from 47 French hospitals. Infect Control Hosp Epidemiol 2007; 28: 1389-95. [ Links ]
46. Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC, Craig WA, Billeter M, Dalovisio JR, Levine DP. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis 2009; 49: 325-7. [ Links ]
47. Sader HS, Jones RN, Gales AC, Silva JB, Pignatari AC; SENTRY Participants Group (Latin America). SENTRY antimicrobial surveillance program report: Latin American and Brazilian results for 1997 through 2001. Braz J Infect Dis 2004; 8: 25-79. [ Links ]
48. Saurina G, Quale JM, Manikal VM, Oydna E, Landman D Antimicrobial resistance in Enterobacteriaceae in Brooklyn, NY: epidemiology and relation to antibiotic usage patterns. J Antimicrob Chemother 2000; 45: 895-8. [ Links ]
49. Schurink CA, Hoitsma M, Rozenberg-Arska M, Joore JC, Hoepelman IM, Bonten MJ. Do cultures contribute to optimisation of antibiotic therapy in the intensive care unit? Int J Antimicrob Agents 2004; 23: 325-31. [ Links ]
50. TEST program (Tigecycline Evaluation and Surveillance Trial). http://www.testsurveillance.com; accessed January 7, 2009. [ Links ]
51. Tognim MC, Andrade SS, Silbert S, Gales AC, Jones RN, Sader HS. Resistance trends of Acinetobacter spp. in Latin America and characterization of international dissemination of multi-drug resistant strains: five-year report of the SENTRY Antimicrobial Surveillance Program. Int J Infect Dis 2004; 8: 284-91. [ Links ]
52. Villegas MV, Lolans K, Correa A, Kattan JN, Lopez JA, Quinn JP. First identification of Pseudomonas aeruginosa isolates producing a KPC-type carbapenem-hydrolyzing beta-lactamase. Antimicrob Agents Chemother 2007; 51: 1553-5. [ Links ]
53. Villegas MV, Lolans K, Correa A, Suarez CJ, Lopez JA, Vallejo M, Quinn JP. First detection of the plasmid-mediated class A carbapenemase KPC-2 in clinical isolates of Klebsiella pneumoniae from South America. Antimicrob Agents Chemother 2006; 50: 2880-2. [ Links ]
54. Warren MM, Gibb AP, Walsh TS. Antibiotic prescription practice in an intensive care unit using twice-weekly collection of screening specimens: a prospective audit in a large UK teaching hospital. J Hosp Infect 2005; 59: 90-5. [ Links ]
Recibido: 15/02/11
Aceptado: 30/05/11














