Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista argentina de microbiología
versión impresa ISSN 0325-7541
Rev. argent. microbiol. vol.43 no.4 Ciudad Autónoma de Buenos Aires oct./dic. 2011
MICROBIOLOGÍA INDUSTRIAL Y AMBIENTAL
Growth response of maize plantlets inoculated with Enterobacter spp., as a model for alternative agriculture
Yolanda E. Morales-García1, Dalia Juárez-Hernández2, Celia Aragón-Hernández3, Miguel A. Mascarua-Esparza1, María R. Bustillos-Cristales1, Luis E. Fuentes-Ramírez1, Rebeca D. Martínez-Contreras1, Jesús Muñoz-Rojas*1
1 Laboratorio de Ecología Molecular Microbiana, Centro de Investigaciones en Ciencias Microbiológicas-Instituto de Ciencias, Benemérita Universidad Autónoma de Puebla, Edifcio 103 J, Ciudad Universitaria, Col. San Manuel, 72570, Puebla, México;
2 Escuela de Biología-Benemérita Universidad Autónoma de Puebla, Edifcio 112-A, Ciudad Universitaria,
3 Laboratorio clínico del ISSSTEP, Av. Venustiano Carranza 810. Col. San Baltazar Campeche, 72550, Puebla, México.
*Correspondence. E-mail: joymerre@yahoo.com.mx
ABSTRACT
A maize rhizosphere isolate was phenotypically and genotypically characterized and identifed as Enterobacter spp. bacterium. Germinated seeds were inoculated, the plantlets were sown in vermiculite and in soil and grown under laboratory and feld conditions, respectively. The adherence, colonization and plant growth promotion capability of Enterobacter sp. UAPS03001 was evaluated in "Rojo-Criollo" maize under laboratory conditions. Twenty days after inoculation, the treated plantlets showed larger biomass than non-inoculated ones. In feld grown plants, the kernel biomass was also greater in inoculated than in non-inoculated plants. The inoculation of maize sprouts with plant growth- promoting bacteria before their sowing in the feld would be an alternative practice for achieving successful yield in temporal agriculture.
Key words: Inoculated plantlets; PGPR; Maize; Enterobacter spp.
RESUMEN
Respuesta de plántulas de maíz inoculadas con Enterobacter spp. como un modelo de agricultura alternativa. En este trabajo se aisló una bacteria de la rizósfera de maíz, que fue caracterizada mediante métodos fenotípicos y genotípicos e identifcada como Enterobacter sp. UAPS03001. La bacteria fue inoculada en semillas de maíz "Rojo-Criollo" germinadas en forma axénica. Las semillas germinadas e inoculadas se plantaron en vermiculita y posteriormente las plántulas fueron cultivadas en vermiculita o en suelo, para evaluar el efecto promotor del crecimiento vegetal de dicha bacteria, bajo condiciones de laboratorio y de campo. Bajo condiciones de laboratorio, también se evaluó la capacidad de esta cepa para adherirse a las plantas de maíz y colonizarlas. Veinte días después de la inoculación, las plántulas inoculadas mostraron una biomasa mayor con referencia a las no inoculadas. En campo, la biomasa de la mazorca fue también mayor en las plantas inoculadas respecto de las plantas no inoculadas. La inoculación de germinados de maíz con una bacteria promotora del crecimiento vegetal y su posterior transferencia a campo podría ser una práctica alternativa para llevar a cabo una producción exitosa en agricultura de temporal.
Palabras clave:Plántulas inoculadas; PGPR; Maíz; Enterobacter spp.
INTRODUCTION
Zea mays (Poaceae) apparently evolved from Teocintle (Zea perennis), (6, 48) in a geographic region that comprises the Central and Southeast regions of Mexico. Its origin as a crop dates back to 5400 (31) to 7000 years BC (32, 33) and was eventually disseminated to other regions in the Americas and the entire world (19, 36). This plant forms the basis of the Mexican diet. For instance, the grains are used for preparing diverse traditional foods and beverages like tortillas, molotes, tamales, gorditas, tostadas, tesgüino, and tejuino (13, 27). Nowadays, maize uses include modern exploitation for producing fructose syrup (46), bio-oil, and others (18). The local "Rojo-Criollo" maize cultivar is used for preparing traditional candies (Secretaría de Turismo de Tlaxcala, México 2010: Tlaxcala pre-hispanicgastronomy, http://www.tlaxcala.gob.mx/turismo/anexo/gastronomico/b_mestiza.html) and a kind of red tortillas. Bacterial inoculation of maize, mainly with Azospirillum, has been a successful common practice (5, 23, 30). Additionally, growth enhancement has also been observed by experimental inoculation with many other bacteria, for instance Pseudomonas (17, 23, 39), Klebsiella pneumoniae, Pantoea agglomerans, Gluconacetobacter diazotrophicus and Herbaspirillum seropedicae (40). Temporal agriculture is the most common farming for maize cultivation in Central Mexico, which is also characterized by poor income. This and other facts have provoked a continuous reduction of traditionally cultivated maize felds (22). One of the greatest problems is related to the erratic start of the rainy season, especially in current times, which is probably due to global climatic change (16, 34, 45). The defciency of water on time and the presence of seed-feeding fauna have forced farmers to invest more in seed (38). In this work, a bacterial strain was isolated from the maize rhizosphere, and was further characterized by phenotypic and genotypic assays. The aim of this work was to inoculate axenic maize seedlings with the isolated bacteria, and to assess their capacity to promote the growth of plants both at growth chamber level and in the feld. The growth of corn plants was stimulated under the conditions explored in this work, so it is proposed that the inoculation of germinated axenic maize sprouts with benefcial bacteria, their subsequent growth, and the introduction of inoculated plantlets to the felds, could be an alternative production system in temporal crops.
MATERIAL AND METHODS
Bacterial isolation and characterization
Strain UAPS03001 was isolated from the rhizosphere of "Rojo Criollo" maize .This variety has been cultivated by some people in San Diego Buena Vista Tlaxcala (Figure 1) for a long time, but, similarly to many other native varieties from Mexico, it has not been genetically characterized yet. Five plants grown for one month were extracted from soil (19° 10' 30.59" N, 98° 09' 50.05" W, Elevation: 2408 MASL) and transported to the laboratory in sterile conditions where soil adhered to their roots (considered rhizosphere) was resuspended in water (1:10 w/v). This mixture was vortexed at 3000 rpm for 3 min and one hundred microliters of serial dilutions (1:10 factor) were plated on Congo Red agar media (37). Bacteria from high dilution were isolated. The strain UAPS03001 was selected for testing its plant growth promoting activity. This strain was characterized by phenotypic characteristics and by 16S rDNA similarity. Biochemical testing was performed with MARK 20 panels from DADE Behring, analyzing the bacterial growth/activity for 24 biochemical tests (including the use of carbon sources) and resistance to 23 antibiotics. Bacterial response was detected with the automated system MicroScan 4 (Baxter Inc. Mexico). The phenotypic tests performed are listed in Table 1. The sequence of the 16S rDNA gene was compared with public sequences. Briefy, genomic DNA was extracted and purifed from bacterial cells grown to stationary phase with a Wizard Genomic DNA Isolation kit (Promega). A fragment of ca. 1365 bp of the 16S rDNA gene was amplifed with the universal primers: UN27F 5'-TAGAGTTTGATCCTGGCTCAG-3' and UN1392R 5'-CAGGGGCGGTGTGTACA-3' (Biodiversa Inc., Mexico). The PCR product was sequenced with the same primers at the Instituto de Biotecnología (UNAM). The sequence was initially analyzed with BLAST, and subsequently with DNA Star. The GenBank accession number of the 16S rDNA sequence of UAPS03001 is HM355806.

Figure 1. Aerial view of San Diego Buenavista (closed in bold line) belongs to "Papalotla de Xicohtencatl" community. It is a place located to the south of Tlaxcala in the Central Mexican Plateau (Altiplano de México). Photo obtained from Google Earth.
Table 1. Phenotypical traits and antibiotic pattern of strain UAPS03001, obtained with the panel MARK 20 from Dade Behring (ID = Enterobacter cloacae with 99.99% of probability)

1 means reference 11, 2 means reference 42, 3 means reference 44. V means variable.
Antibiotic resistance
Antibiotic resistance was tested for designing media for the reisolation of the inoculated strain. UAPS03001 was plated on LB and Congo Red media (37). Antibiotic multidiscs (Bio Rad, Mexico) were placed in duplicate on inoculated agar plates.
Germination of maize seeds
Seeds of the "Rojo-Criollo" maize variety were germinated as follows: the seeds were washed with distilled water and rinsed with 70% ethanol for 10 min and immersed under agitation in 6.5% sodium hypochlorite for 20 min. The seeds were washed eight times with sterile distilled water under sterile conditions, and incubated for three days at 30°C under high environmental humidity on MS solid medium (29) supplemented with 20 mM glucose and 30 mM sucrose (medium MSJ3). Seeds showing microbial growth after incubation were discarded.
Growth promotion experiment in environmental chamber
Strain UAPS03001 was grown overnight in LB broth. Bacterial growth was inoculated in the same medium and grown until stationary phase. The cells were washed and resuspended in the same volume in sterile distilled water. Cell quantity was determined in triplicate with the drop plate method (12). Forty germinated maize seeds were immersed in the bacterial suspension for 1 h and sown in tubes containing 5 g of sterile vermiculite. As a control, other forty germinated maize seeds were immersed in sterile distilled water under the conditions mentioned above. The sprouts were grown under the following conditions: 25 to 28°C, light/darkness cycle 16/8 h, 70% humidity. The plantlets were watered once with 25 ml of MS solution without carbon source (medium MSJ1).
a) Quantifcation of bacterial adherence. The number of cells adhering to the sprouts was quantifed after 12 h. Six replicates and six controls were vigorously vortexed in a volume of sterile distilled water. Cell numbers were determined by the drop plate method on agar selective media.
b) Quantifcation of bacterial colonization. Ten and 20 days after inoculation, the roots of ten plants for each treatment were immersed in 20 ml of sterile distilled water and vigorously vortexed for 40 s. Rhizospheric bacteria were determined with serial dilutions of the bacterial suspension by dropping 20 µl on selective media plates and 24-hour incubation.
c) Aerial plant biomass. The aerial region of 20 plants was chopped into small pieces, dehydrated at 70°C for four days, and weighed. Data were compared with the Student's t-test.
Field experiment
Plantlet production. Two hundred "Rojo-Criollo" maize variety seeds were germinated in MSJ3 medium as explained above. One-half of the sprouts was inoculated with bacterial cells as mentioned whereas the other half was treated as control. The sprouts were seeded in sterile vermiculite and watered with MSJ1 as described above. The plantlets were incubated for fve days under the conditions of the previous experiment and subsequently transferred to the feld.
Plant growth . Sowing was at the start of the rainy season in May 2007. The feld, located in Papalotla, Tlaxcala, Mexico (19° 10' 19.93" N, 98° 09' 52.74" W, Elevation: 2394 MASL), was ploughed and the plantlets were sown with 0.8 m of distance between them, placing 10 plants by furrow, with a total of 10 rows for each treatment. The space between rows was 70 cm and each experimental block was in a space of 10 m in length (distance from the furrow) by 8 m wide. The border between inoculated and non-inoculated plantlets was 3 m in length. Holes, ca. 15 cm in depth and 20 cm in diameter were made with the aid of a spade. The plantlets were introduced in the holes (one plantlet per hole), covered with soil, and watered with 500 ml distilled water. Two days later the plantlets were watered as before. One month after seeded, each plant was fertilized with 7.5 g of NPK 17:17:17 (containing 1.7 g of nitrogen, 1.7 g of P2 O5 , and1.7 g of K2O per 10 g) (15), and a fnal plough was made. Eight month-old plants were harvested and the maize kernels were weighed.
Data Analysis
Statistical analysis for rhizospheric colonization and aerial dry biomass was performed using Sigma Plot (Handel Scientifc Software). Differences were considered according to the Student´s t test results.
RESULTS
Isolation and characteristics of rhizospheric bacteria
The strain designated UAPS03001 was isolated from the rhizosphere of "Rojo-Criollo"maize. The morphology of this strain in Congo Red agar corresponded to small red circular translucent colonies, showing regular margin, convex elevation and smooth texture. Phenotypic tests of strain UAPS03001 showed 95% similarity to Enterobacter cloacae (Table 1) and the sequence comparison of the 16S rDNA gene showed 96.3% identity with the same species (Table 2). This strain was resistant to some antibiotics (Table 3), and ampicillin (Ap) and erythromycin (E) were chosen for the selective media added with Ap (50µg/ml) and E (50 µg/ml). Bacterial growth was comparable in selective and non-selective media (results not shown).
Table 2. Sequence similarity with 16S rRNA of Enterobacteriaceae species [% identity]
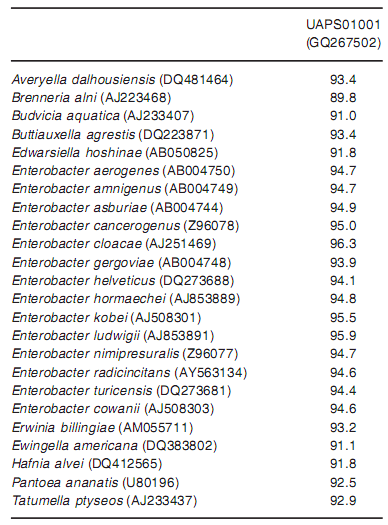
The accession number of each bacterial species is shown in parentheses and Enterobacter species identities in bold letters.
Table 3. Resistance of Enterobacter spp. to antibiotics from Multidiscs Biorad

Adherence and colonization assays
Adherence and colonization assays were developed in triplicate with similar results for each independent experiment. We are showing results for one of them. The sprouts were inoculated with a suspension containing 9 × 108 CFU/ml, showing an adherence of 1.05 × 107 CFU per plant (SD 7 × 106 ). The bacterial population in the rhizosphere 10 days after inoculation (dai) was 7.12 ×108 CFU/g vermiculite and at 20 days had increased to 1.86 × 109 UFC/g vermiculite (Fig. 2). Several microbial morphologies, different from those of strain UAPS03001 were detected from non-inoculated plantlets. Those microorganisms were recovered in low quantities (1 × 103 CFU/g vermiculite) and were not further characterized.

Figure 2. Enterobacter spp. UAPS03001 cell numbers colonizing maize rhizosphere. Quantifcations were done at 10 and 20 days after inoculation (dai) in selective medium. Mean of ten replicates and standard deviations are presented. Differences were statistically signifcant with the Student's t-test (p≤0.05).
Growth promotion experiments both in environmental chamber and in the feld
Adherence and colonization assays were developed in triplicate with similar results for each independent experiment. We are showing results for one of them. The inoculated plants showed statistically signifcant greater biomass than the controls (Figure 3) 0.168 g (±0.034) for inoculated plants and 0.080 g (±0.049) for non-inoculated plants. All the plants survived and showed strong vigor throughout the experiment (Results not shown). In inoculated plants, the fowering started ca. 200 days after transplantation. The total kernel biomass of eight month-plants, obtained from 100 plants of each treatment, was higher in inoculated than in non-inoculated plants, 19.6 and 12.86 kg, respectively.

Figure 3. Aerial dry biomass of maize plants at twenty days after inoculation. Dark grey bar, non-inoculated controls; grey bar, inoculated plants. Mean of twenty plants and standard deviations are presented. Differences were statistically signifcant with the Student's t test (p≤0.05).
DISCUSSION
The strain UAPS03001 isolated from the rhizosphere of "Rojo-Criollo" maize was designated as Enterobacter spp. because phenotypic tests of the strain showed 95% similarity to Enterobacter cloacae (Table 1) and the sequence comparison of the 16S rDNA gene showed high identity value (96.3%) with the same species (Table 2). However, according to polyphasic taxonomy, more studies are needed to designate the species name (47). Enterobacter spp. has also been isolated from the rhizosphere of other plants, e.g. from Lolium perenne rhizosphere (41), rice rhizosphere (26) and from soil near the roots of leguminous plants (49), showing its natural association to this environment. It is desirable to fnd native strains for inoculation attempts. The frst advantage would be the adaptation to local biological and non-biological conditions, whereas the second advantage would be the use of indigenous organisms that would prevent environmental disturbance.
The strain herein isolated showed good adherence to seed sprouts and root colonization capability, similar to other rhizospheric bacteria (3, 4, 8, 28). Inoculation experiments were performed under environmental chamber conditions, where non-inoculated plants showed low quantities of other microorganisms different from strain UAPS03001 after 20 days. This means that a natural bacteria population is present inside the maize seed, which could not be eliminated by superfcial sterilization, as suggested by Bresan and Borges (2). The inoculated plants showed statistically signifcant greater biomass than the controls under environmental chamber conditions and the total kernel biomass of eight month plants was higher in inoculated than in non-inoculated plants. Other experiments have demonstrated growth promotion by some Enterobacter strains on different plants, i. e. stimulation of the growth of tomato, pepper and mung bean plants by the plant growth-promoting bacterium Enterobacter cloacae CAL3 (25) or the stimulation of the growth of Brassica oleracea by the nitrogen-fxing endophytic bacteria Enterobacter spp. strain 35 (50). In the present work, the mechanisms for promoting plant growth by Enterobacter spp. are not defnite but as in other growth promoting bacteria, N-fxation, production of IAA and cytokinins, phosphate solubilization, and phytopathogen antagonism could participate in this activity (24), as has been suggested for Enterobacter ludwigii (41) and Enterobacter radicincitans (14).
Temporal agriculture is dependent on climate, so it is also highly susceptible to even minor environmental alterations (16). Particularly, plant germination is extremely vulnerable to lack of water. Most of the inoculation experiments have been done with seeds, so the bacterial survival capability in the soil had been determinative for plant colonization (1, 43). In this work, the inoculation was done in sprouts instead, in an attempt to increase bacterial and plant survival. Under our conditions, Enterobacter spp. UAPS03001 was profcient at promoting the growth of inoculated maize sprouts. Even though some Enterobacter strains have been designed as benefcial bacteria given their capability to suppress plant disease (20) and their biocontrol properties (21), some species of Enterobacter have been reported as opportunistic human pathogens (10). Hence, the results presented in this work have to be taken only as a model of study of inoculated plantlets, because they could be a latent danger when applied extensively. More studies are needed to determine potential hazards or benefts of Enterobacter spp. UAPS03001. At the present time, other benefcial bacteria such as Azospirillum brasilense could result more suitable to that end (5, 8, 24, 30). Sprout inoculation with benefcial bacteria as an alternative to seed inoculation could be advantegeous to maize sown in temporal agriculture and could possibly ameliorate foraging by seed-feeding fauna (9, 38). In this work, the maize seeds were germinated in MSJ3 medium, but less expensive substrates, such as vermiculite, could also be used. The scaling-up of this procedure for application in productive farms is a problem for maize but has been currently developed in other crops such as strawberry (7). Nevertheless, the process can be used for green house plantlet production without scaling-up. This procedure of inoculation in other crops is under test in our lab. A future variant of particular interest is the remediation of polluted temporal soils with plants inoculated via sprouts, for instance, with P. putida KT2440, which is able to use a variety of carbon sources (35), including xenobiotic compounds.
Acknowledgements: We are indebted to Dr. Michael Dunn for constructive English corrections, and Dr. Alfredo Torres for valuable opinions on the work. Jesus Muñoz-Rojas and Yolanda Elizabeth Morales-García were partially supported by PROMEP/103.5/09/1852 (BUAP-PTC-116) and by VIEP-BUAP MURJNAT10G.
1. Bashan Y. Inoculants of plant growth-promoting bacteria for use in agriculture. Biotechnol Adv 1998; 16: 729-70. [ Links ]
2. Bressan W, Borges MT. Delivery methods for introducing endophytic bacteria into maize. Biocontrol 2004; 49: 315-22. [ Links ]
3. Böltner D, Godoy P, Muñoz-Rojas J, Duque E, Moreno-Morillas S, Sánchez L, Ramos JL. Rhizoremediation of lindane by root-colonizing Sphingomonas. Microb Biotechnol 2008; 1: 87-93. [ Links ]
4. Caballero-Mellado J, Martínez-Romero E, Estrada de los Santos P, Fuentes-Ramírez LE. Maize colonization by Acetobacter diazotrophicus. In: Elmerich C, Kondorosi A, Newton WE, editors. Biological Nitrogen Fixation for the 21st Century. Paris, Kluwer Academic Publishers, 1998, p. 381-2. [ Links ]
5. Dobbelaere S, Croonenborghs A, Thys A, Ptacek D, Vanderleyden J, Dutto P, Labandera-Gonzalez C, Caballero-Mellado J, Aguirre JF, Kapulnik Y, Brener S, Burdman S, Kadouri D, Sarig S, Okon Y. Responses of agronomically important crops to inoculation with Azospirillum. Aust J Plant Physiol 2001; 28: 871-9. [ Links ]
6. Dorweiler J, Stec A, Kermicle J, Doebley J. Teosinte glume architecture 1: a genetic locus controlling a key step in maize evolution. Science 1993; 262: 233-5. [ Links ]
7. Durner EF, Poling EB, Maas JL. Recent advances in straw-berry plug transplant technology. Hort Technol 2002; 12: 545-50. [ Links ]
8. Fallik E, Okon Y, Fischer M. Growth response of maize roots to Azospirillum inoculation: effect of soil organic matter content, number of rhizosphere bacteria and timing of inoculation. Soil Biol Biochem 1988; 20: 45-9. [ Links ]
9. Fanadzo M, Chiduza C, Mnkeni PNS. Comparative performance of direct seeding and transplanting green maize under farmer management in small scale irrigation: a case study of Zanyokwe, Eastern Cape, South Africa. Afr J Agric Res 2010; 5: 524-31. [ Links ]
10. Flores-Tena FJ, Guerrero-Barrera AL, Avelar-González FJ, Ramírez-López EM, Martínez-Saldaña MC. Pathogenic and opportunistic gram-negative bacteria in soil, leachate and air in San Nicolás landfll at Aguascalientes, México. Rev Lat Microbiol 2007; 49: 25-30. [ Links ]
11. Grimont F, Grimont PAD. The genus Enterobacter. In: Dworkin M, editor. The Prokaryotes, 3rd edn. Singapore, Springer Science, Business Media, Inc., 2006, p. 197-211. [ Links ]
12. Herigstad B, Hamilton M, Heersink J. How to optimize the drop plate method for enumerating bacteria. J Microbiol Methods 2001; 44: 121-9. [ Links ]
13. Hernández XE. Maize and man in the Great Southwest. Econ Bot 1981; 39: 416-30. [ Links ]
14. Kampfer P, Ruppel S, Remus R. Enterobacter radicincitans sp nov., a plant growth promoting species of the family Enterobacteriaceae. Syst Appl Microbiol 2005; 28: 213-21. [ Links ]
15. Kanyajua SM, Ayaga GO. Phosphate sources at planting. En: Sitawa-Ogutu JK, Mwariri M, editors. A guide to choice of mineral fertilisers in Kenya. Technical Note Series N° 17. KARI Headquarters ©Kenya Agricultural Research Institute, 2006, p. 9-11. [ Links ]
16. Köchy M. Effects of simulated daily precipitation patterns on annual plant populations depend on life stage and climatic region. BMC Ecol 2008; 8: 1-23. [ Links ]
17. Kumar B, Trivedi P, Pandey A. Pseudomonas corrugata: A suitable bacterial inoculant for maize grown under rainfed conditions of Himalayan region. Soil Biol Biochem 2007; 39: 3093-100. [ Links ]
18. Lawrence CJ, Harper LC, Schaeffer ML, Sen TZ, Seigfried TE, Campbell DA. MaizeGDB: the maize model organism database for basic, translational, and applied research. Int J Plant Genomics 2008; 2008: 496957. [ Links ]
19. Lia VV, Confalonieri VA, Ratto N, Cámara Hernández JA, Miante Alzogaray AM, Poggio L, Brown TA. Microsatellite typing of ancient maize: insights into the history of agriculture in southern South America. Proc Biol Sci 2007; 274: 545-54. [ Links ]
20. Liu S, Hu X, Lohrke SM, Baker J, Buyer JS, de Souza JT, Roberts DP. Role of sdhA and pfkA and catabolism of reduced carbon during colonization of cucumber roots by Enterobacter cloacae. Microbiol 2007; 153: 3196-209. [ Links ]
21. Lohrke SM, Dery PD, Li W, Reedy R, Kobayashi DY, Roberts DP. Mutation of rpiA in Enterobacter cloacae decreases seed and root colonization and biocontrol of damping-off caused by Pythium ultimum on cucumber. Mol Plant Microbe Interact 2002; 15: 817-25. [ Links ]
22. López-Martínez JD, Salazar-Sosa E. Comparison of maize genotypes under defcient soil moisture conditions. Terra 1998; 16: 331-5. [ Links ]
23. Lugtemberg B, Kamilova F. Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 2009; 63: 541-56. [ Links ]
24. Lucy M, Reed E, Glick R. Applications of free living plant growth-promoting rhizobacteria. Antonie Van Leeuwenhoek 2004; 86: 1-25. [ Links ]
25. Mayak S, Tirosh T, Glick BR. Stimulation of the growth of tomato, pepper and mung bean plants by the plant growthpromoting bacterium Enterobacter cloacae CAL3. Biol Agric Hortic 2001; 9: 261-74. [ Links ]
26. Mehnaz S, Mirza MS, Haurat J, Bally R, Normand P, Bano A, Malik KA. Isolation and 16S rRNA sequence analysis of the benefcial bacteria from the rhizosphere of rice. Can J Microbiol 2001; 47: 110-7. [ Links ]
27. Miguel MA, Arellano-Vázquez JL, García de los Santos G, Miranda-Colín S, Mejía-Contreras JA, González-Cossío FV. Maize landraces of Chalqueño race blue kernel. Agronomic traits and seed quality. Rev Fitotec Mex 2004; 27: 9-15. [ Links ]
28. Molina L, Ramos C, Duque E, Ronchel MC, García JM, Wyke L, Ramos JL. Survival of Pseudomonas putida KT2440 in soil and in the rhizosphere of plants under greenhouse and environmental conditions. Soil Biol Biochem 2000; 32: 315-21. [ Links ]
29. Murashige T, Skoog F. A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Plant 1962; 15: 473. [ Links ]
30. Okon Y, Labandera-González CA. Agronomic applications of Azospirillum: An evaluation of 20 years worldwide feld inoculation. Soil Biol Biochem 1994; 26: 1591-601. [ Links ]
31. Piperno DR, Flannery KV. The earliest archaeological maize (Zea mays L.) from highland Mexico: new accelerator mass spectrometry dates and their implications. Proc Natl Acad Sci USA 2001; 98: 2101-3. [ Links ]
32. Piperno DR, Moreno JE, Iriarte J, Holst I, Lachniet M, Jones JG, Ranere AJ, Castanzo R. Late Pleistocene and Holocene environmental history of the Iguala valley, central Balsas watershed of Mexico. Proc Natl Acad Sci USA 2007; 104: 11874-81. [ Links ]
33. Pohl MED, Piperno DR, Pope KO, Jones JG. Microfossil evidence for pre-Columbian maize dispersals in the neotropics from San Andrés, Tabasco, México. Proc Natl Acad Sci USA 2007; 104: 6870-5. [ Links ]
34. Porter R, Semenov MA. Crop responses to climatic variation. Philos Trans R Soc Lon B Biol Sci 2005; 360: 2021-35. [ Links ]
35. Ramos JL, Krell T, Segura A, Duque E. Responses of Pseudomonas to small toxic molecules by a mosaic domains. Curr Opin Microbiol 2009; 12: 215-20. [ Links ]
36. Rebourg C, Chastanet M, Gouesnard B, Welcker C, Dubreuil P, Charcosset A. Maize introduction into Europe: the history reviewed in the light of molecular data. Theor Appl Genet 2003; 106: 895-903. [ Links ]
37. Rodríguez-Cáceres EA. Improved medium for isolation of Azospirillum spp. Appl Environ Microbiol 1982; 44: 990-1. [ Links ]
38. Romero-Balderas KG, Naranjo EJ, Morales HH, Nigh RB. Daños ocasionados por invertebrados silvestres al cultivo de maíz en la selva lacandona, Chiapas, México. Interciencia 2006; 31: 276-83. [ Links ]
39. Rosas SB, Avanzini G, Carlier E, Pasluosta C, Pastor N, Rovera M. Root colonization and growth promotion of wheat and maize by Pseudomonas aurantiaca SR1. Soil Biol Biochem 2009; 41: 1802-6. [ Links ]
40. Riggs PJ, Chelius MK, Iniguez AL, Kaeppler SM, Tripplett EW. Enhanced maize productivity by inoculation with diazotrophic bacteria. Aust J Plant Physiol 2001; 28: 829-36. [ Links ]
41. Shoebitz M, Ribaudo CM, Pardo MA, Cantore ML, Ciampi L, Cura JA. Plant growth promoting properties of a strain of Enterobacter ludwigii isolated from Lolium perenne rhizosphere. Soil Biol Biochem 2009; 41: 1768-74. [ Links ]
42. Sthephan R, Trappen SV, Cleenwerck I, Vancanneyt M, De Vos P, Lehner A. Enterobacter turicensis sp. nov. and Enterobacter helveticus sp. nov., isolated from fruit powder. Int J Syst Evol Microbiol 2007; 57: 820-6. [ Links ]
43. Streeter JG. Effect of trehalose on survival of Bradyrhizobium japonicum during desiccation. J Appl Microbiol 2003; 95: 484-91. [ Links ]
44. Swanson EC, Collins MT. Use of the API 20E system to identify veterinary Enterobacteriaceae. J Clin Microbiol 1980; 12: 10-4. [ Links ]
45. Tilman D, Lehman C. Human-caused environmental change: impacts on plant diversity and evolution. Proc Natl Acad Sci USA 2001; 98: 5433-40. [ Links ]
46. Van Twisk P, Meltzer BW, Cormack RH. Production of glucose from maize grits on commercial scale. Starch-Stärke 1976; 28: 23-5. [ Links ]
47. Vandamme P, Pot B, Guillis M, De Vos P, Kersters K, Swings J. Polyphasic taxonomy, a consensus approach to bacterial systematics. Microbiol Rev 1996; 60: 407-38. [ Links ]
48. Wang H, Nussbaum-Wagler T, Li B,BL, Zhao Q, Vigouroux Y, Faller M, Bomblies-Yant K, Lukens L, Doebley J. The origin of the naked grains of maize. Nature 2005; 436: 714-9. [ Links ]
49. Yoon SJ, Choi YJ, Min HK, Cho KK, Kim J, Lee SC, Jung YH. Isolation and identifcation of phytase-producing bacterium, Enterobacter sp. 4, and enzymatic properties of phytase enzyme. Enzyme Microbial Technol 1996; 18: 449-54. [ Links ]
50. Zakria M, Ohsako A, Saeki Y, Yamamoto A, Akao S. Colonization and growth promotion characteristics of Enterobacter sp. and Herbaspirillum sp. on Brassica oleracea. Soil Sci Plant Nutr 2008; 54: 507-16. [ Links ]
Recibido: 15/03/2011
Aceptado: 31/08/2011














