Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista argentina de microbiología
versión impresa ISSN 0325-7541
Rev. argent. microbiol. vol.44 no.2 Ciudad Autónoma de Buenos Aires abr./jun. 2012
ARTICULO ORIGINAL
Grape stalks as substrate for white rot fungi, lignocellulolytic enzyme production and dye decolorization
Laura Levin*, Luis Diorio, Emanuel Grassi, Flavia Forchiassin
Laboratorio de Micologia Experimental, Departamento de Biodiversidad y Biologia Experimental, Facultad de Ciencias Exactas y Naturales, PROPLAME-PHRIDEB, CONICET, Universidad de Buenos Aires. Ciudad Universitaria, Pabellón II, Piso 4, (1428) Ciudad Autònoma de Buenos Aires, Argentina.
*Correspondence. E-mail: lale@bg.fcen.uba.ar
ABSTRACT
The aim of this work was to evaluate the potential of grape stalks, an agroindustrial waste, for growth and lignocellulolytic enzyme production via solid-state fermentation, using the following three white rot fungi: Trametes trogii, Stereum hirsutum and Coriolus antarcticus. The decolorization of several dyes by the above mentioned cultures was also investigated. Similar values of dry weight loss of the substrate were measured after 60 days (33-43 %). C. antarcticus produced the highest laccase and Mn-peroxldase activities (33.0 and 1.6 U/g dry solid). The maximum endoglucanase production was measured in S. hirsutum cultures (10.4 U/g), while the endoxylanase peak corresponded to T. trogii (14.6 U/g). The C. antarcticus/grape stalk system seems potentially competitive in bioremediation of textile processing effluents, attaining percentages of decolorization of 93, 86, 82, 82, 77, and 58 % for indigo carmine, malachite green, azure B, remazol brilliant blue R, crystal violet and xylidine, respectively, in 5 h.
Key words: Grape stalks; Solid state fermentation; White rot fungi; Lignocellulolytic enzymes; Dye degradation
RESUMEN
Uso del escobajo como sustrato para el crecimiento de hongos de la pudrición blanca, la producción de enzimas ligninolíticas y la decoloración de tinturas. El objetivo de este trabajo fue evaluar el potencial del escobajo, un residuo agroindustrial, como sustrato para el crecimiento y la producción de enzimas lignocelulósicas de tres hongos causantes de pudrición blanca en la madera: Trametes trogii, Stereum hirsutum y Coriolus antarcticus. Para ello se utilizaron técnicas de fermentación en estado sólido. También se ensayó la decoloración de colorantes industriales sobre estos cultivos. La pérdida de peso seco del sustrato fue similar después del día 60 (33-43 %). C. antarcticus produjo las mayores actividades de lacasa y Mn-peroxidasa (33,0 y 1,6 U/g peso seco). La mayor actividad endoglucanasa fue medida en cultivos de S. hirsutum (10,4 U/g), y la mayor actividad endoxilanasa en T. trogii (14,6 U/g). El sistema C. antarcticus/escobap mostró un importante potencial para su aplicación en la biorremediación de efluentes textiles, con porcentajes de decoloración de 93, 86, 82, 82, 77 y 58 % para índigo carmín, verde de malaquita, azure B, azul R brillante de remazol, cristal violeta y xilidina, respectivamente, en 5 h.
Palabras clave: Escobajo; Fermentación en estado sólido; Hongos de la pudrición blanca; Enzimas lignocelulósicas; Decoloración
Argentina is a leading wine-producing country in the world. The quality and productivity of its wineries are very competitive compared with those of more traditional wine- producing countries such as France, Italy and Spain. Given its production output, Argentina rates as the main Latin American producer and has a significant growth potential. The Argentine Wine Production Statistics Institute stated that during 2009, crop yields reached approximately 2,137,000 tons of grapes and wine production was about 1,076 million liters (17). This picture also shows the need for adopting a sustainable management of the resources employed and for recycling the important amount of residues produced. Disposal and landfill of those wastes present environmental and social drawbacks. New technologies were proposed not only for their reuse in agriculture, but also for the production of common and novel products for other sectors (31).
Pomace is the main solid residue of wine production, which contains 45 % grape skins, 30 % seeds and 25 % grape stalks. It constitutes 12 % of a fresh grape's weight. In Argentina, it accounts for around 213,000 tons/y (17). Grape stalks have a high degree of fibers [lignin (22.94 %), cellulose (29.95 %) and hemicellulose (35.33 %)] and a high percentage of nutritive mineral elements, especially nitrogen and potassium (25). The use of grape stalks in the form of single cell protein after solid state fermentation (SSF) has been proposed as ruminant feed or feeding component (32). Results indicate that, after biological lignin removal, the cellulose is better accessible to rumen microorganisms, and due to its good protein value and low lignin content, it has a similar value of digestibility as forages (5460 %). SSF is another way of producing a variety of compounds like ethanol, citric acid, gluconic acid, carotenoids, xanthan, etc. (31).
Enzyme production is a growing field of biotechnology. Most enzyme manufacturers produce enzymes using submerged fermentation (SmF) techniques. In general, enzyme titers in SSF are higher than in SmF, when comparing the same strain and fermentation broth (39). White rot fungi (WRF) are so far unique in their ability to completely degrade all components of lignocellulosic materials. Cellulose biodégradation is a synergistic process involving endo-B-1,4-glucanase, cellobiohydrolase and B-glucosidase. Hydrolysis of xylans mainly requires the action of endo-B-1,4-xylanase and B-xylosidase. The capability to degrade lignin is due to their extracellular nonspecific and nonstereoselective enzyme system composed by laccases, lignin peroxidases (LiPs) and Mn-peroxidases (MnPs), which function together with H202-producing oxidases and secondary metabolites (5, 10, 26). The same unique nonspecific mechanisms that give these fungi the ability to degrade lignin also allow them to degrade a wide range of pollutants, among them: polycyclic aromatic hydrocarbons, chlorinated phenols, polychlorinated biphenyls, dioxins, pesticides, explosives and dyes (35, 44). Purified laccases, LiPs and MnPs of different fungi are able to decolorize dyes of different chemical structure (16, 19, 34, 35). All of these enzymes are industrially important; therefore organisms able to produce them are interesting in view of the potential importance in industrial processes such as bioremediation, biobleaching of pulp paper, degradation and detoxification of recalcitrant substances or in the food industry. Thus, the efficient production of these enzymes in a low-cost medium is interesting for biotechnological applications. Moreover, the value-added conversion of the bioproducts from winemaking can help in reducing the negative costs and demonstrating sustainability in winemaking (31).
Various agricultural substrates/byproducts and WRF have been used successfully in SSF for ligninolytic enzyme production (39). Two closely related substrates were assayed in SSF for lignocellulolytic enzyme production: grape seeds, as substrate for laccase production by Trametes hirsuta (28, 30) and grapevine cutting wastes as substrate for laccase, MnP, cellulose and xylanase production by Cerrena unicolor{\ 3). Grape stalks were assayed as substrate for ligninolytic enzyme production by Phanerochaete chrysospohum during semi-solid-state cultivation. Grape stalks cultures of this fungus led to a decolorization of around 70 % of Poly R-478 after 8 days of dye incubation (41). Grape stalks added to submerged cultures of Trametes versicolor induced laccase production. Supernatants of T. versicolor cultured in such substrate proved their capacity to decolorize phenol red (25) and indigo carmine (27). But as far as we know, grape stalks had never been assayed before as sole substrate for the production of lignocellulolytic enzymes by WRF in SSF.
Several studies have demonstrated the ability of WRF to decolorize synthetic dyes. However, most studies on dye decolorization have been carried out using liquid culture conditions or solid cultures on agar plates, which, however, do not reflect the natural living conditions (e.g. in wood and other lignocellulosic substrates) of WRF. SSF was chosen here because it mimics the natural environment of the WRF. Textile dye decolorization has been demonstrated with cell suspensions, immobilized cells, crude enzymatic extracts and purified ligninolytic enzymes (1, 12, 16, 19, 20). The major drawback to using an enzyme preparation is that once the enzymes become inactivated, decolorization activity ceases. However, with a whole cell culture, the enzymes could be continually replenished. Immobilized cultures tend to have a higher level of activity and are more resilient to environmental perturbations such as pH, or exposure to toxic chemical concentrations than suspension cultures (33, 38). Immobilization by encapsulation in a matrix such as alginate may be too costly for wastewater treatment, while surface immobilization on an inexpensive material such as woodchips or lignocellulosic wastes is cheaper (43).
Trametes trogii (BAFC 463), Coriolus antarcticus (BAFC 266), and Stereum hirsutum (BAFC 2234) are Argentinean strains of WRF that had proved to be efficient ligninolytic enzyme producers in previous studies (21, 23, 24, 30). Their ability to decolorize a wide range of textile dyes has been recently demonstrated (12, 16, 20, 22, 23, 29). The aim of this work was to evaluate the potential of grape stalks as support for their growth and lignocellulolytic enzyme production under solid state conditions. The in vivo and in vitro decolorization of several dyes, by the above mentioned cultures was also investigated in order to assess their degradative abilities.
MATERIALS AND METHODS
Fungal strain and culture conditions
Trametes trogii (BAFC 463; MYA 28-11), S. hirsutum (BAFC 2234) and C. antarcticus (BAFC 266) (Basidiomycota) were obtained from the Centro de Colecciones de la Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires (BAFC), and maintained at 4°C on ME agar (malt extract 1.2 %, glucose 1 %, agar 2 %) slants. Incubation was carried out at 28 °C under stationary conditions in 100 ml Erlenmeyer flasks for up to 60 days. Grape stalks cut into pieces of 3-5 cm [15 g per Erlenmeyer (dry basis)] was used as solid substrate for cultures, moistened with 20 ml of distilled water and 5 ml mycelial suspension (inoculum) (initial moisture content 65 %). The medium was autoclaved for 20 min at 121 °C, and aseptically inoculated with the mycelial suspension.To prepare the inoculum, Erlenmeyer flasks containing ME medium were inoculated with one agar plug (25 mm2) cut out from the margin of a 5-day-old colony grown on ME agar and incubated for 5 days at 28 °C and the mycelium obtained was blended in three cycles of 15 s. For all experiments, measurements were carried out in triplicate parallel cultures. The values are reported as the mean with a standard deviation of (SD) less than 10 %.
Sample preparation
Weight losses were determined on the basis of the initial and final dry weights, drying the content of each flask to constant weight at 80 °C. Enzyme extraction: crude extracts were obtained by adding 5 ml of distilled water per g wet solid, to the contents of each flask, stirring for 20 min, followed by centrifugation. The supernatants were stored at -20 °C until enzyme determination.
Enzyme assays
Lacease activity (E.C:1.10.3.2) was measured with 2,2'-azino bis (3-ethylbenzthiazoline-6-sulphonicacid) (ABTS) in 0.1 M sodium acetate buffer (pH 3.6). Oxidation of ABTS was determined by the increase in A^ (£420= 36/mM cm) (8). Mn-peroxidase activity (MnP) (E.C:1.11.1.13) was measured using phenol red as thesubstrate in 0.1 M sodium dimethylsuccinate buffer (pH 4.5) (e610 = 22/mM cm) (14). Lignin peroxidase activity (LiP) (E.C:1.11.1.14) was assayed by the azure B method (2). Ligninolytic activities were measured at 30 'C. Endo-B-D-1,4-glucanase (E.C:3.2.1.4), and endo-p-D-1,4-xylanase (E.C:3.2.1.8) activities were determined by measuring the reducing sugars released from carboxymethyl cellulose or oat xylan, respectively, as substrates, in 50 mM sodium acetate buffer, pH 4.8, at 50°C. Liberated reducing sugars were quantified by the Somogyi-Nelson method (45), using either glucose or xylose as standards. International enzymatic units (U) were used (umol/min).Enzymatic activities in the extracts recovered from the solid-state cultures were reported in U/ml. In terms of production, the activity was defined as U/g dry residue (substrate plus mycelium) (U/g).
In vivo decolorization experiments
The reaction mixture contained in 1 g wet material (substrate plus fungus, 40 days post-inoculation) in 10 ml of different dyes (concentration 100 uM). Six dyes belonging to five different classes were analyzed. Decolorization was determined by measuring the decrease of dye absorbance at its maximum visible wavelength 505, 588, 592, 608, 618, and 650 nm, respectively for: xylidine [acid red 26: azoic dye, CI (colour index N.°): 16150], remazol brilliant blue R (RBBR, reactive blue 19: anthraquinone dye, CI 61200), indigo carmine (acid blue 74: indigoic dye, CI 73015), malachite green (basic green 4: triphenylmethanic dye, CI 42000) and crystal violet (gentian violet: triphenylmethanic dye, CI 42555), and azure B (heterocyclic dye, CI 52010). The initial pH of the solutions was 5.2 for all dye systems. An abiotic control (substrate without fungus) was conducted in parallel. A blank treatment containing no dye was used as a control to evaluate possible color changes due to water extractives liberated by the fungal colonized substrate. Results are expressed as percentage of dye decolorization after 5 h.
In vitro decolorization experiments
The decolorization capacity of the cell-free extracts was also evaluated. The reaction was carried out in test tubes at 30 °C.The reaction mixture contained 1 ml of crude filtrate, and either 20 uM of malachite green or 30 uM of the other dyes assayed. Boiled extracts were used as controls.
RESULTS AND DISCUSSION
Growth characteristics: dry weight loss and reducing sugars
Time course of reducing sugars and dry weight loss of the substrate are shown in Figure 1. Loss in total dry weight in the substrate was measured after 60 d of fermentation (33-43 %). Data for reducing sugars, produced by enzymatic substrate hydrolysis showed that on day 40 they had been almost completely metabolized.
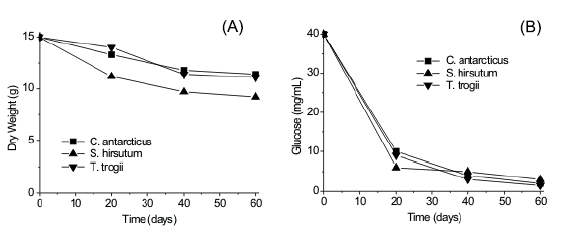
Figure 1. Time course of dry weight loss (A) reducing sugars (mg/ml of aqueous extract) (B) when culturing T. trogii, C. antarcticus and S. hirsutum on grape stalks.
Enzyme activities
The time course of lignocellulolytic enzyme production by T. trogii, C. antarcticus and S. hirsutum, in SSF using grape stalks as substrate was studied. Extracts from cultures were assayed to determine the activities of cellulases, xylanases, and ligninases. Figure 2 illustrates the lignocellulolytic enzyme profiles. Laccase (Figure 2A), MnP (Figure 2B), endoglucanase (Figure 2C) and endoxylanase (Figure 2D) were measured. Attempts to detect LiP were unsuccessful. If produced in this medium, LiP levels could be too low to be detected (2). On the other hand, the extraction procedure may not have been appropriate to guarantee the recovery of the enzymes adsorbed on the substrate (46). LiP activity had been previously detected in another T. trogii strain (11). Regarding ligninases, the highest laccase activity (33 U/g) was produced by C. antarcticus after 40 d. On that same date, this fungus also produced the peak of MnP (1.62 U/g); afterwards both activities decreased sharply. Maximum endoglucanase titers were produced by S. hirsutum after 60 d (10.44 U/g). The highest endoxylanase levels (14.56 U/g) were detected in T. trogii after 20 d of cultivation, but this activity decreased to virtually zero on day 60 post-inoculation. Endoglucanase production by T. trogii followed a similar pattern. Laccase activity of T. trogii as well as endoglucanase and endoxylanase activities of S. hirsutum, and C. antarcticus endoglucanase activity reached their peak value on the last sampling day. All these activities, with the exception of the endoglucanase activity from S. hirsutum, showed a lineal increase from day 20 to the last fermentation day. Therefore, higher enzyme activities might have been achieved if the cultivation time had been prolonged. Ligninolytic systems of WRF are mainly activated during the secondary metabolic phase of the fungus (5). But the three fungi growing in SSF, which were assayed in this study, produced hydrolases along with oxidases also during trophophase in parallel with growth (taking into account that on day 20, glucose was not depleted from the medium), showing that these enzymes may not be secondary metabolites.

Figure 2. Time course of laccase (A), MnP (B), endoglucanase (C) and endoxylanase (D) production by T. trogii, C. antarcticus and S. hirsutum in solid state cultures, grown on grape stalks. Means of three independent experiments with a SD less than 10 % are shown.
Previously, lignocellulosic substrates had shown to efficiently induce high amounts of ligninolytic enzymes in other white rot basidiomycetes, for example some of the highest records of laccase production were obtained in Coriolopsis rigida (108 U/g) (15) and T. hirsuta (68.4 U/g) (40), both fungi grown on barley bran.The deuteromycete Aspergillus awamori produced 40.4 U/g of endoxylanase and 9.6 U/g of endoglucanase when grown in SSF on grape pomace (7). Although the activities attained in this work are lower than those recorded in such substrates, this is the first report proving the feasibility of using grape stalks as substrate for SSF lignocellulolytic enzyme production by WRF. Because of its low cost, worldwide abundance and the resulting high levels of enzyme production, the SSF using grape stalks as principal substrate could be used to scale up the production of Ngnocellulases.
Dye decolorization
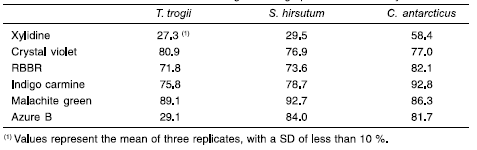
The cultures of the three fungi demonstrated potential to decolorize a broad spectrum of chemically different dyes. All the dyes tested were decolorized to some extent, with varying percentages of decolorization (Table 1). Forty-day-old cultures of C. antarcticus showed the highest ability to decolorize the different dyes assayed (in coincidence with maximal laccase and MnP activities determined), attaining percentages of decolorization of about 93, 86, 82, 82, 77, and 58 % for indigo carmine, malachite green, azure B, RBBR, crystal violet and xylidine, respectively, in 5 h. Decolorization was due to two simultaneous effects: the physical process of adsorption on the mycelium, and the enzymatic degradation caused by the ligninolytic enzymes produced by the fungi. Decolorization due to adsorption on the support was negligible, as determined with the abiotic control. Extracting dyes (with alcoholic extraction solution) from heavily colonized substrates for quantification proved difficult. Nevertheless, the role of adsorption in dye decolorization appears to be minimal. The dyes were rapidly removed from the medium, as a result of the physical adsorption process, but they were later eliminated both from the solution and the mat surfaces, as a consequence of the enzymatic degradation. Similar results were obtained recently, when adsorption of a textile dye by dead biomass pellets of T. versicolor was determined and compared with dye removal by enzymatic degradation (4), while adsorption demonstrated to be important in dye decolorization by Pestalotiopsis guepinii (42).
Table 1. Degree of decolorization (%/ 5h) of xylidine, crystal violet, remazol brilliant blue R (RBBR), indigo carmine, malachite green and azure B (100 uM) by T. trogii, S. hirsutum and C. antarcticus solid state cultures grown on grape stalks for 40 days

The results obtained in this work are in coincidence with previous decolorization studies applying these three strains. The ability of S. hirsutum to decolorize and detoxify indigo carmine, xylidine and malachite green under SSF conditions using either: wheat bran, soya bran or a mixture of both (1:1), had been previously investigated (29). Seventeen-day cultures on soya bran showed the highest decolorization values: 68 % for xylidine (20 uM) after 120 min of contact time, 90 % for malachite green (10 uM) after 90 min. Cultures on wheat bran/soya bran showed to be more efficient to degrade indigo carmine (20 uM) (95 % after 30 min). Polyacrylamide gel electrophoresis revealed a correspondence between decolorization bands and laccase activity bands; thus, laccase activity could be associated with the process of dye decolorization. Detoxification assays were carried out, using the extraction liquid from malachite green degradation. Detoxification of malachite green was up to 100 % after 2 h of contact time (29). T. trogii (BAFC 463) is an outstanding producer of laccase. In an optimized SSF wood-based medium supplemented with malt extract, peptone and copper, it produced up to 901 U/g of laccase and 20 U/g of MnP (21). While in SmFI trogii extracellular fluids which rendered the highest ligninolytic production (45.32 U/ml laccase, 0.21 U/ml MnP) also showed the greatest ability to decolorize the dyes xylidine, malachite green and anthraquinone blue at rates of 2.14; 1.35 mg and 3 mg dye/I x h, respectively (22). Eighteen day-old C. antarcticus cultures on ME medium were able to decolorize 28 -100 % of five different dyes added in an hour, representing decolorization rates of 5.3, 3.9, 6.6, 22.5, and 22.5 mg/l x h of malachite green, RBBR, xylidine, indigo carmine and Poly R-478, respectively (23). The different rates reflect different capacities of the cultures to remove dyes with diverse chemical structures. Indigo carmine is a dye, which was shown to be easily decolorized by different wood rotting fungi. It can be degraded either by purified laccase, LiP or MnP (16, 34, 36, 37) Jarosz-Wilkoazka et al. (18) also demonstrated that anthraquinonic dyes were decolorized by fungi easier and faster than azoic dyes. A low efficiency of decolorization of some azoic dyes compared to other dye types was also reported for P. chrysosporium and T. versicolor (49). Microorganisms do not readily degrade azoic dyes. Sulpho and azoic groups do not occur naturally, thus sulphonated azoic dyes are recalcitrant to biodégradation. Biodegrada-bility of azoic dyes depends on the presence of very specific changes in their molecular structure. Anthraqui-none, azoic and indigoic dyes were decolorized by the laccase of T. versicolor; however, the mechanism of laccase-catalyzed decomposition was different depending on dye structures. Anthraquinone was directly oxidized by the laccase, azoic and indigo dyes were not the substrates of laccase and small molecule metabolites mediated the interaction between the dyes and the enzyme. The decolorization rate of nonsubstrate dyes was limited by the concentration of mediating compounds rather than by laccase activity in the solutions (47). When screening dye decolorizing abilities of WRF, azure B is among the most recalcitrant dyes. This dye is used to detect LiP activity because it is not oxidized by laccase or MnP alone, but it was partially decolorized in the presence of the mediators (2, 9). With the addition of the natural mediator p-coumaric acid, about 60 % decolorization was achieved in 2 h with Trametes villosa laccase (9). The azure B decolorization ability ofFlavodon flavus was tested in ME broth; about 50 % decolorization was achieved after 24 h (36). In the present work, C. antarcticus cultures decolorized 82 % of this dye after 5 h. Decolorization rates obtained in the present study were comparable to or even exceeded those previously reported for the fastest dye-degrading WRF, among them: T. versicolor and P. chrysosporium. C. antarcticus capacity to decolorize the azo dye xylidine (6.6 mg/l x h) is similar to that previously described for T. versicolor in relation to different azo dyes (3 mg/l x h (6) and 5 mg/l x h (48)), and is comparable to that of T. versicolor to decolorize RBBR (3-7 mg/l x h (6)). Moreover, its ability to decolorize azure B is outstanding.
The crude extracellular extracts were able to decolorize some of these dyes but not as efficiently as the cultures (Table 2). In a previous work, when T. versicolor was grown in a submerged culture with glucose as carbon source and the addition of grape stalks, it produced 400 U/l laccase and 36 U/l MnP (27). Sixty-two percent decolorization of phenol red (75 uM) after 72 h was observed when applying the ligninolytic fluids obtained in the abovementioned cultures (25), while indigo carmine (60 uM) was almost completely decolorized after 48 h by the extracellular fluids from T. versicolor grown under the same conditions (27). In our study, the best decolorization was obtained for indigo carmine (74 % after 2 h). Similarly, while Pleurotus ostreatus decolorized 12 of 23 industrial dyes when grown on solid media, the crude extracellular extracts were able to decolorize only 5 dyes, showing that other enzymatic mechanisms could be involved in dye decolorization in vivo experiments (37). Although laccase and MnP were detected in cell-free extracts of the three fungi assayed, probably H202 or other necessary factors involved in the catalytic cycle of the enzymes were lacking. These enzymes and their associated components such as H202 are present extracellularly, but in biobleaching studies with T. versicolor, Archibald (3) has shown that they must be constantly replenished from active biomass. Mycelial presence becomes significant if decolorization requires such biomass-associated factors.
Table 2. Degree of decolorization (%/ 2h) of malachite green, 20 uM, xylidine, crystal violet, remazol brilliant blue R (RBBR), indigo carmine, and azure B, 30 uM, by T. trogii, S. hirsutum and C. antarcticus cell-free extracts from cultures grown on grape stalks for 40 days
In conclusion, grape stalks appear to be a good choice when searching for lignocellulosic materials to immobilize cultures of WRF for decolorization purposes. The three fungi assayed colonized it very readily producing lacease and MnP activities, the biomass was not easily sloughed off, its integrity was maintained over a long period of time and decolorization rates of a broad spectrum of dyes were high. Moreover, the utilization of this material also helps to solve pollution problems caused by its disposal. Considering the magnitude of the decolorization obtained, the C. antarcticus/ grape stalk system may be employed in the bioremediation of colored effluents without the help of any mediator.
Acknowledgements: the authors are grateful to Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina), Universidad de Buenos Aires for financial support and Ing. Jorge A. Ivanissevich from Mendoza Province, for supplying the grape stalks.
1. Anastasi A, Spina F, Prigione V, TiginiV, Giansanti P, Varese GC. Scale-up of a bioprocess for textile wastewater treatment using Bjerkandera adusta. Bioresour Technol 2010; 101:3067-75. [ Links ]
2. Archibald FS. A new assay for lignin-type peroxidases employing the dye Azure B. Appl Environ Microbiol 1992; 58:3110-6. [ Links ]
3. Archibald FS. The role of fungus-fiber contact in the biobleaching of kraft brownstock by Trametes (Coriolus) versicolor. Holzforschung 1992; 4: 305-10. [ Links ]
4. Aretxaga A, Romero S, Sarra M, Vicent T. Adsorption step in the biological degradation of a textile dye. Biotechnol Progress 2001; 17: 664-8. [ Links ]
5. Blanchette RA. Degradation of the lignocellulose complex in wood. Can J Bot 1995; 73: 999-1010. [ Links ]
6. Borchert M, Libra JA. Decolorization of reactive dyes by the white rot fungus Trametes versicolor in sequencing batch reactors. Biotechnol Bioeng 2001; 75: 313-21. [ Links ]
7. Botella C, de Ory I, Webb C, Cantero D, Blandino A. Hydrolytic enzyme production by Aspergillus awamori on grape pomace. Biochem Eng J 2005; 26:100-6. [ Links ]
8. Bourbonnais R, Paice MG, Reid ID, Lanthier P, Yaguchi M. Lignin oxidation by laccase isozymes from Trametes versicolor and role of the mediator 2,2'-azino-bis (ethylbenzothiazoline-6-sulfonic acid) in Kraft lignin depolimerization. Appl Environ Microbiol 1995; 61:1876-80. [ Links ]
9. Camarero S, Ibarra D, Martinez MJ, Martinez AT. Lignin-derived compounds as efficient laccase mediators for decolorization of different types of recalcitrant dyes. App Environ Microbiol 2005; 71:1775-84. [ Links ]
10. Dashtban M, Schraft H, Syed TA, Qin W. Fungal biodégradation and enzymatic modification of lignin. Int J Biochem Mol Biol 2010; 1:36-50. [ Links ]
11. Dhouib A, Hamza M, Zouari H, Mechichi T, Hmidi R, Labat M, Martinez MJ, Sayadi S. Screening for ligninolytic enzyme production by diverse fungi from Tunisia. World J Microbiol Biotechnol 2005; 21:1415-23. [ Links ]
12. Diorio LA, Mercuri AA, Nahabedian DE, Forchiassin F. Development of a bioreactor system for the decolorization of dyes by Coriolus versicolor f. antarcticus. Chemosphere 2008; 72:150-6. [ Links ]
13. Elisashvili VI, Daushvili LP, Zakariashvili NG, Kachlishvili ET, Kiknadze MO, Tusishvili KA. Effect of supplementary carbon sources and exogenous phenolic compounds on the lignocellulolytic system of Cerrena unicolor during the solid-state fermentation of grapevine cutting wastes. Microbiology 1998;67:3-37. [ Links ]
14. Glenn JK, Gold MH. Purification and characterisation of an extracellular Mn(ll)-dependent peroxidase from the lignin degrading basldiomycete Phanerochaete chrysosporium. Arch Biochem Biophys 1985; 242: 329-41. [ Links ]
15. Gômez J, Pazos M, Rodriguez Couto S, Sanromân MA. Chesnut shell and barley bran as potential substrates for laccase production by Coriolopsis rigida under solid-state conditions. J Food Eng 2005; 68:315-9. [ Links ]
16. Grassi E, Scodeller P, Filiel N, Carballo R, Levin L. Potential of Trametes trogii culture fluids and its purified laccase for the decolorization of different types of recalcitrant dyes without the addition of redox mediators. Int Biodeterior Biodegrad 2011 ; 65: 635-43. [ Links ]
17. Instituto Nacional de Vitivlnicultura. Disponible en http:// www.inv.gov.ar/
18. Jarosz-Wilkolazka A, Kochmanska-Rdest J, Malarczyk E. Fungi and their ability to decolourize azo and anthraquinonic dyes. Enzyme Microb Technol 2002; 30: 566-72.
19. Kokol V, Doliska A, Eichlerova I, Baldrian P, Nerud F. Decolorization of textile dyes by whole cultures of Ischnoderma resinosum and by purified laccase and Mn-peroxidase. Enzyme Microb Technol 2007; 40: 1673-77. [ Links ]
20. Levin L, Melignani E, Ramos AM. Effect of nitrogen sources and vitamins on ligninolytic enzyme production by some white-rot fungi. Dye decolorization by selected culture filtrates. Bioresour Technol 2010; 101: 4554-63. [ Links ]
21. Levin L, Herrmann C, Papinutti L. Optimization of lignocellulolytic enzyme production by the white-rot fungus Trametes trogii in solid-state fermentation using response surface methodology. Biochem Eng J 2008; 39:207-14. [ Links ]
22. Levin L, Forchiassin F, Viale A. Ligninolytic enzyme production and dye decolorization by Trametes trogii: application of the Plackett-Burman experimental design to evaluate nutritional requirements. Process Biochem 2005; 40:1381-7. [ Links ]
23. Levin L, Papinutti L, Forchiassin F. Evaluation of Argentinean white rot fungi for their ability to produce lignin-modifying enzymes and decolorize industrial dyes. Bioresour Technol 2004; 94:169-76. [ Links ]
24. Levin L, Forchiassin F, Ramos AM. Copper induction of lignin-modifying enzymes in the white-rot fungus Trametes frog//. Mycologia 2002; 94:377-83. [ Links ]
25. Lorenzo M, Moldes D, Rodríguez Couto S, San román MA. Improving lacease production by employing different lignocellulosic wastes in submerged cultures of Trametes versicolor. Bioresour Technol 2002; 82: 109-13. [ Links ]
26. Martínez T, Ruiz-Dueñas FJ, Martínez MJ, del Río JC, Gutiérrez A. Enzymatic delignification of plant cell wall: from nature to mill. Curr Opin Biotechnol 2009; 20: 348-57.
27. Moldes D, Lorenzo M, Sanromán MA. Different proportions of lacease isoenzymes produced by submerged cultures of Trametes versicolor grown on lignocellulosic wastes. Biotechnol Lett 2004; 26: 327-30.
28. Moldes D, Gallego PP, Rodriguez Couto S, Sanromán MA. Grape seeds: the best lignocellulosic waste to produce lacease by solid state cultures of Trametes hirsuta. Biotechnol Lett 2003; 25:491 -5. [ Links ]
29. Mouso N, Levin L, Salariato DL, Papinutti VL, Forchiassin F. Decoloración y detoxificación con Stereum hirsutum, basidiomicete de pudrición blanca de la madera. En: Herkovits J, editor. Salud ambiental y humana: una visión holística. Buenos Aires, SETAC Press, 2006, p. 153-8.
30. Mouso N, Papinutti L, Forchiassin F. Efecto combinado del cobre y pH inicial del medio de cultivo sobre la producción de lacasa y manganeso peroxidasa por Stereum hirsutum (Willd) Pers. Rev Iberoam Micol 2003; 20:176-8. [ Links ]
31. Nerantzis ET, Tataridis P. Integrated enology-utilization of winery by-products into high added value products. e-JST 2006; 1:179-89.
32. Nicolini L, Volpe C, Pezzotti A, Carilli A. Changes in in-vitro digestibility of orange peels and distillery grape stalks after solid-state fermentation by higher fungi. Bioresour Technol 1993; 45:17-20.
33. Perullini M, Jobbágy M, Mouso N, Forschiassin F, Bilmes SA. Silica-alginate-fungi biocomposites for remediation of polluted water. J Mater Chem 2010; 20: 6479-83. [ Links ]
34. Podgornik H, Poljansek I, Perdih A. Transformation of indigo carmine by Phanerochaete chrysosporium ligninolytic enzymes. Enzyme Microb Technol 2001; 29: 166-72. [ Links ]
35. Pointing SB. Feasibility of bioremediation by white-rot fungi. Appl Microbiol Biotechnol 2001; 57: 20-33. [ Links ]
36. Raghukumar C, D'SouzaTM, Thorn RG, Reddy CA. Lignin-modifying enzymes of Flavodon flavus, a basidiomycete isolated from a coastal marine environment. Appl Environ Microbiol 1999;65:2103-11. [ Links ]
37. Rodriguez E, Pickard MA, Vazquez-Duhalt R. Industrial dye decolorization by laceases from ligninolytic fungi. Current Microbiol 1999; 38:27-32. [ Links ]
38. Rodriguez Couto S. Dye removal by immobilised fungi. Biotechnol Adv 2009; 27:227-35. [ Links ]
39. Rodríguez-Couto S, San Román MA. Application of solid state fermentation to ligninolytic enzyme production. Biochem Eng J 2005; 22: 211-9.
40. Rodríguez-Couto S, Rosales C, Gundín M, Sanromán MA. Exploitation of a waste from the brewing industry for lacease production by two Trametes species. J Food Eng 2005; 64:423-8. [ Links ]
41. Rodríguez-Couto S, Rodríguez R, Gallego PP, Sanromán MA. Biodégradation of grape cluster stems and ligninolytic enzyme production by Phanerochaete chrysosporium during semi-solid-state cultivation. Acta Biotechnol 2003; 23:65-74.
42. Saparrat M, Nazareno C, Hammer E. Decolorization of synthetic dyes by the deuteromycete Pestalotiopsis guepinii CLPS no. 786 strain. J Basic Microbiol. 2006; 46:28-33. [ Links ]
43. Shin M, Nguyen T, Ramsay J. Evaluation of support materials for the surface immobilization and decoloration of amaranth by Trametes versicolor. Appl Microbiol Biotechnol 2002; 60: 218223. [ Links ]
44. Singh H, editor. Mycoremediation, Fungal Bioremediation, 1st edition. Hoboken, NJ, John Wiley & Sons Inc, 2006, p. 1 -592.
45. Somogyi MJ. Notes on sugar determination. J Biol Chem 1952; 195:19-23. [ Links ]
46. Souza-Cruz P, Freer J, Siika-Aho M, Ferraz A. Extraction and determination of enzymes produced by Ceriporiopsis subvermispora during biopulping of Pinus taeda wood chips. Enzyme Microb Technol 2004; 34: 228-234. [ Links ]
47. WongYYu J. Laccase-catalyzed decolorization of synthetic dyes. Water Res 1999; 33: 3512-3520. [ Links ]
48. Yang FC, Yu JT. Development of a bioreactor system using immobilized white rot fungus for decolorization. Part I: cell immobilization and repeated-batch decolorization tests. Bioprocess Eng 1996; 15: 307-310. [ Links ]
49. Young L, Yu J. Ligninase-catalyzed decolorization of synthetic dyes. Water Res 1997; 31: 1187-93. [ Links ]
Recibido. 12/7/2011 - Aceptado: 6/2/2012














