Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista argentina de microbiología
versión impresa ISSN 0325-7541
Rev. argent. microbiol. vol.45 no.1 Ciudad Autónoma de Buenos Aires mar. 2013
MICROBIOLOGÍA CLÍNICA Y ENFERMEDADES INFECCIOSAS
Serotype distribution of pneumococci isolated from pediatric patients with acute otitis media and invasive infections, and potential coverage of pneumococcal conjugated vaccines
Vanesa Reijtman1, Sofía Fossati2, Claudia Hernández1, Patricia Sommerfleck3, Patricia Bernáldez3, Mirta Litterio1, Griselda Berberian4, Mabel Regueira3, Horacio Lopardo1*
1Servicio de Microbioiogía,
3Servicio de Otorrinoiaringoiogía,
4Servicio de Controi Epidemioiógico e infectoiogía, Hospitai de Pediatría "Prof. Dr. Juan P. Garrahan", Combate de ios Pozos 1881 (1245) Ciudad Autónoma de Buenos Aires;
2Instituto Nacionai de Enfermedades infecciosas, ANLiS "Dr. Carios G. Maibrán", Ciudad Autónoma de Buenos Aires, Argentina.
*Correspondence. E-maii: hiopardo@garrahan.gov.ar
ABSTRACT
A 16-month prospective, descriptive study was conducted on pneumococcal serotype distribution isolated from children with acute otitis media (AÜM) and invasive infections (INV). Eighty-nine children with pneumococcal INV and 324 with a first episode of AOM were included. Bacterial pathogens (N = 326) were isolated from the middle-ear fluid of 250 patients. A total of 30 pneumococcal serotypes were identified. Prevalent serotypes were 14, 19A, 9V, 3, 19F, 6A, 23F, and 18C in AOM and 14, 1, 19A, 5, 12F, 6B, and 18C in INV. Potential coverage with PCV10 vaccine would be 46.5 % and 60.7 % for pneumococci involved in AOM and INV, respectively; it would be 71.7 % and 73 % with PCV13. PCV10, conjugated with a Haemophilus protein, would have an immunologic coverage of 39.9 % for AOM vs. 18.5 % with PCV13. However, differences in the prevention of INV were crucial for the decision to include the 13-valent vaccine in the national calendar for children less than two years old in Argentina.
Key words: Streptococcus pneumoniae; Serotype; Pediatrics; Acute otitis media; Invasive infections; Vaccines.
RESUMEN
Distribución de serotipos de neumococos aislados de pacientes pediátricos con otitis media aguda e infecciones invasivas y su cobertura potencial a través de vacunas conjugadas. Se realizó un estudio prospectivo descriptivo sobre la distribución de serotipos de neumococos aislados de niños con otitis media aguda (OMA) y con infecciones invasivas (INV) en un período de 16 meses. Se incluyeron 89 niños con INV neumocócicas y 324 con un primer episodio de OMA. Trescientos cuarenta y seis patógenos se aislaron de las secreciones de oído medio obtenidas de 250 pacientes. Se identificaron 30 serotipos y los más prevalentes fueron el 14, 19A, 9V, 3, 19F, 6A, 23F y 18C en OMA y el 14, 1, 19A, 5, 12F, 6B y 18C en INV. La cobertura potencial con la vacuna PCV10 sería de 46,5 % y 60,7 % para neumococos involucrados en OMA y en INV, respectivamente; con la PCV13, esta sería de 71,7 % y 73 %. La PCV10 conjugada con una proteína de Haemophilus tendría una cobertura inmunológica del 39,9 % para OMA, contra una cobertura del 18,5 % de la PCV13. Sin embargo, las diferencias en la prevención de INV fueron determinantes a la hora de considerar incorporarla al calendario nacional de vacunación para niños menores de 2 años en la Argentina.
Palabras clave: Streptococcus pneumoniae; Serotipos; Pediatría; Otitis media aguda; Infecciones invasivas; Vacunas.
INTRODUCTION
Pneumococcal pneumonia is considered one of the most frequent causes of childhood mortality in developing countries (12). Streptococcus pneumoniae is a major cause of pneumonia and of other invasive infections, such as bacteremia and meningitis, or less severe but prevalent infections, such as acute otitis media (AOM). The existence of more than 90 immunologically distinct serotypes complicates the design of effective vaccines eliciting responses to polysaccharide capsules. However, a 23-valent capsular vaccine for preventing pneumococcal diseases in adults and children over two years of age has been available for several years. Introduction of a heptavalent pneumococcal conjugate vaccine (PCV7), containing 4, 6B, 9V, 14, 18C, 19F, and 23F serotypes, into immunization programs for infants of several countries has had a major impact on invasive pneumococcal disease incidence in young children (11).
Recently, two new vaccines have been launched in our country: a 10-valent Haemophilus-protein-polysaccharide (PCV-10), including PCV7 and 1, 5 and 7F serotypes, and a 13-valent conjugated (PCV-13) vaccine, adding serotypes 3, 6A and 19A to PCV10.
The 10-valent pneumococcal vaccine may also prevent AOM episodes due to non-typeable Haemophilus influenzae, provided that it is a Haemophilus protein D-conjugated vaccine (7). This occasion prompted us to analyze the prevalent serotypes of pneumococci obtained from the middle ear of pediatric patients with acute otitis media (AOM), and to compare these results with those obtained from blood, joint, cerebrospinal fluid, and other sterile sites obtained from pediatric patients with invasive infections (INV). In addition, we analyzed the potential coverage of pneumococcal serotypes producing AOM and INV by the conjugated vaccines available in Argentina.
MATERIALS AND METHODS
A prospective, descriptive study on serotype distribution of pneumococci isolated from pediatric patients with AOM and INV was conducted from May 2, 2009 to August 31, 2010 at the Hospital de Pediatría "Prof. Dr. Juan P. Garrahan", a tertiary care pediatric hospital in Buenos Aires, Argentina. The potential coverage of different conjugated vaccines was analyzed. Clinical findings and prevalence of other pathogens in AOM have been published elsewhere (9).
Children with AOM were evaluated using otomicroscopy. When purulent effusion retained in the middle ear was observed, tympanocentesis and culture of middle-ear fluid (MEF) were performed. Immunocompromised patients and those with chronic otitis media were excluded from the study.
Samples of AOM were stored in an oxygen-free atmosphere device (Tab, Laboratorios Britania, Ciudad Autónoma de Buenos Aires, Argentina) until their initial culture. Cultures were performed in sheep blood agar and in thioglycolate broth (both incubated at 35 °C in air) and in chocolate agar plates (incubated at 35 °C in 5 % CO2). Anaerobic cultures were routinely performed in blood agar plates + vitamin K and anaerobic broth and incubated in an anaerobic jar. Samples from patients with invasive infections were processed following conventional methods.
Serotyping was performed with an initial screening against a latex reagent (Copenhagen Seruminstitut, Denmark) and then confirmed using the quellung reaction at the Instituto Nacional de Enfermedades Infecciosas (INEI) of the ANLIS "Dr. Carlos G. Malbran", the reference center for pneumococcal serotyping in Argentina. Antisera were obtained from the Copenhagen Seruminstitut, Denmark. Only serotype 6C was identified by a polymerase chain reaction method (http//www.cdc.gov/ncidod/biotech/strp/pcr.htm).
Penicillin susceptibility tests were performed using the Etest (AB Biodisk, Solna, Sweden). Breakpoints were those recommended by CLSI for oral penicillin V (susceptible: MIC < 0.06 ug/ml, intermediate: MIC between 0.125 and 1 ug/ml, and resistant: MIC> 2 ug/ml) (1).
RESULTS
Eighty-nine pediatric patients (median age months, range 0-120 months) with pneumococcal INV and 324 with a first episode of AOM diagnosed by otolaryngologists were included. They represented the whole population assisted in the hospital with INV or AOM fulfilling the inclusion criteria.
INV included bacteremia (N = 45), pneumonia (N = 29), meningitis (N = 4), arthritis (N = 4), cellulitis (N = 3), peritonitis (N = 3) and lung cyst (N = 1). Most isolates were obtained from blood cultures (N = 71), while only 8 were isolated from pleural fluid, 4 from cerebrospinal fluid, 3 from joint fluid, and one each from abdominal fluid, lung biopsy and orbital punction. Pneumococcal serotypes found in the different INV are listed in Table 1.
Table 1. Serotype distribution among pneumococci isolated from Argentinean pediatric patients with different invasive infections
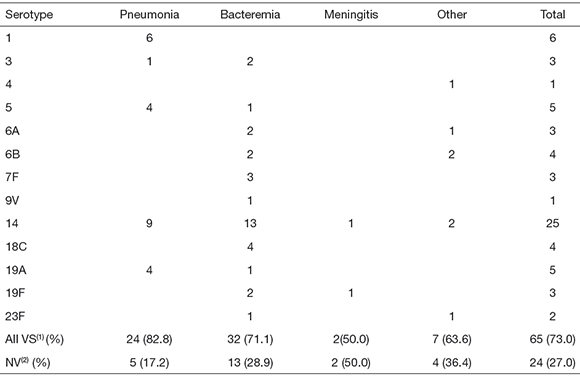
Other: cellulitis (N = 3), arthritis (N = 4), peritonitis (N = 1) and lung cyst (N = 1). (1)VS: vaccine serotypes, (2)NV: non-vaccine serotypes.
Four hundred and thirty-three samples were obtained from these 324 patients with AOM. Of these patients, 180/324 (55.6 %) were male and 144/324 (44.4 %) were female. Median age was 8 months (range, 1-120 months). Bilateral AOM was recorded in 109/324 (33.6 %) children. One hundred and twenty (37 %) of the 324 children have received antimicrobial treatment prior to the diagnosis of AOM while none of them have received the PCV-7 vaccine. Negative cultures were found in 74/324 (22.8 %) children. Different bacterial isolates simultaneously grew from samples of MEF obtained from one or both ears (mixed cultures) in 71/324 (21.9 %) patients.
Three hundred and twenty-six bacterial pathogens were isolated from the middle-ear fluid of 250 patients. Streptococcus pneumoniae (N = 133, 76 in single and 57 in mixed cultures) and H. influenzae (N = 122, 70 in single and 52 in mixed cultures) were the most prevalent species in AOM (40.8 % and 37.4 %, respectively). One hundred and twenty-seven pneumococci were used for the present study, as six isolates were not available at the time of serotyping. Most isolates of H. influenzae were non-typeable (N=119), and the other three were type a, type b, and type d.
Eighty-nine pneumococci were isolated from the same number of children with INV during the same period.
A total of 30 pneumococcal serotypes were identified, being serotype 14 the most prevalent both in AOM and in INV (Table 2). Other frequent serotypes were 19A, 9V, 19F, and 3 in AOM and 1, 5, 12F, 19A, 18C, and 6B in INV.
Table 2. Serotype distribution among pneumococci isolated from Argentinean pediatric patients with acute otitis media (AOM) or invasive infections (INV). Potential coverage of different conjugated vaccines
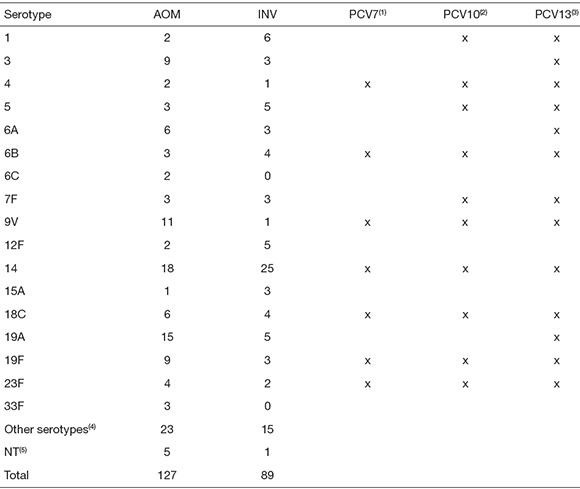
|1|PCV7, 7-valent pneumococcal conjugated vaccine; |2|PCV10, 10-valent pneumococcal conjugated vaccine; (3)PCV 13, 13-valent pneumococcal conjugated vaccine. <4>Other serotypes from AOM: 7C (1), 8 (1), 9N (1), 15C (1), 17F (2), 18A (1), 22F (1), 23A (1), 23B (2), and other non-vaccinal serotypes (12); other serotypes from INV: 9N (1), 10A (1), 11A (2), 15B (1), 15C (1), 17F (1), 22F (2), 23B (1), 33C (1), and other non-vaccinal serotypes (4); (5)NT: non-typeable.
Considering only children younger than 2 years old, we observed that most frequent serotypes were 14 (36.6 %) 12F (9.8 %), 3 (7.3 %), and 7F (4.9 %) among 41 INV, and 14 (15.8 %), 19A (10.5 %), 9V (9.6 %), 19F (7 %), and 3 (7 %) among 114 AOM.
Taking into account only the available isolates from AOM, 91 (71.7 %) of S. pneumoniae isolates were susceptible, 34 (26.8 %) were intermediate and 2 (1.5 %) were resistant to penicillin (MIC> 2ug/ml). Percentages of penicillin-susceptible and penicillin-intermediate isolates from INV were 56 (62.9 %) and 33 (37.1 %), respectively.
Serotypes most frequently associated with diminished susceptibility to penicillin were 14, 19A, 6A, 33F and 9V (Table 3).
Table 3. Penicillin susceptibility of different serotypes of pneumococci obtained from Argentinean pediatric patients with acute otitis media (AOM) or invasive infections (INV)
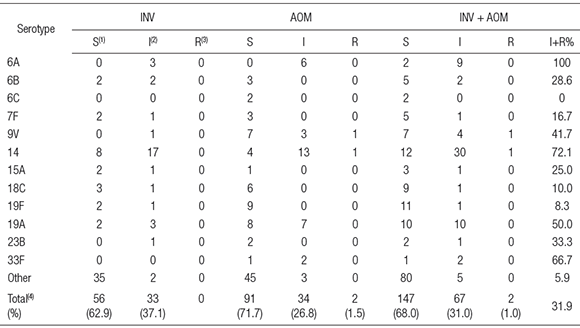
(1)S = susceptible (MIC < 0.06 ug/ml), (2)I = intermediate (0.125> MIC < 1 ug/ml), (3)R = resistant ( MIC> 2 ug/ml); (4)non-statistically significant differences between INV and AOM (p> 0.1, 95 % confidence interval) (Chi-square test, Graph pad Prism, version 5.03).
DISCUSSION
Potential coverage of different vaccines was 40.2 % and 44.9 % with PCV7, 46.5 % and 60.7 % with PCV10, and 71.7 % and 73 % with PCV13, for pneumococci involved in AOM and INV, respectively. Though prevalent serotypes differ from previous national studies (see below), potential coverage calculated for children less than 15 years of age using data from the SIREVA program for Argentina (Sistema Regional para Evaluación de Vacunas) would be quite similar for INV: 39.3 % with PCV7, 71.3 % with PCV10 and 82.9 % with PCV13 (6).
The capsular 23-valent vaccine, containing antibodies for 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17, 18C, 19A, 19F, 20, 22F, 23F and 33F may prevent up to 92 % of INV according to other reports (6). In our experience the 23-valent vaccine may prevent 88.8 % of INV due to S. pneumoniae. As it is not immunogenic or effective in children under two years of age, pediatric pneumococcal vaccines based on capsular polysaccharides conjugated to carrier proteins have been developed.
The highest coverage with PCV7 for INV of young children has been reported in the USA, Canada and Australia (80 - 90 %), followed by Europe and Africa (70 - 75 %), Latin America (around 65 %, with less than 40 % for Argentina) and finally Asia (approximately 50 %) (3, 6).
To date, 7-valent, 10-valent, and 13-valent conjugated pneumococcal vaccines have been licensed for use in Argentina; however, only the latter has recently been included in the national immunization calendar.
The main objective of these vaccines is to prevent INV, but as AOM is a prevalent disease in children under two years of age, some authors highlight the advantage of the 10-valent pneumococcal vaccine. They consider that such vaccine may also prevent AOM episodes due to non-typeable H. influenzae, provided that it is a Haemophilus protein D-conjugated vaccine (7). One hundred and twenty-five children (39.9%) experienced episodes of AOM due to serotypes of S. pneumoniae included in the PCV10 (N = 39), to H. influenzae (N = 70) or to both (N = 14). As the PCV10 vaccine is conjugated with a Haemophilus protein, we may speculate that it would have an immunologic coverage of 39.9% for AOM episodes vs. 17.9% with PCV13 and 12% with PCV7 (p < 0.05) (Table 3)(7).
The present study was designed to assess the prevalent serotypes of both pneumococci isolated from children with INV and from non-immunocompromised AOM over a 15-month period in a tertiary care pediatric hospital in Argentina.
Our figures are quite different from those obtained in other countries, and differences were also found between INV and AOM serotypes within this study. Comparing them with pneumococcal serotypes isolated from other Latin American countries, we found that serotype 14 was the most frequent serotype in Dominican Republic, Peru, Paraguay, Panama, Ecuador, Costa Rica, Colombia, Chile, and Brazil but not in Uruguay, Venezuela, Mexico and several coutries of Central America. Serotypes 1, 19A and 6B were among the seven most prevalent serotypes in Venezuela, Paraguay, Nicaragua, Colombia, and Chile (6)
The most prevalent serotypes were 14, 19A, 9V, 3, 19F, 6A, 23F, and 18C in AOM and 14, 1, 19A, 5, 12F, 6B, and 18C in INV. There were three AOM-prevalent serotypes and four INV-prevalent serotypes that are not included in the 7-valent vaccine, respectively. Only one of them (12F) is not included in the 13-valent vaccine, while 19A (AOM and INV), 3 and 6A (only AOM) are not included in the 10-valent vaccine.
Argentinean data obtained in 2009, in which serotypes 14, 1, 5, 19A, 3, 7F, 6B and 9V were found to be the most prevalent in children with INV, have some differences with our results, probably due to the different number of studied isolates or because of geographic variations (6). Differences between INV and AOM may be due to the different clonal distribution found while analyzing macrolide-resistant isolates (8).
INV serotypes were quite different from our own historical records (year 2000, 81 isolates), when most prevalent serotypes were 14 (35.8 %),1 (9.9 %), 23F (7.4 %), 7F (7.4 %), 6A (4.9 %), 6B (3.7 %) and 3 (3.7 %). The absence of serotype 19A in 2000 is especially remarkable (Casimir L, Ceinos MC, Hernández C. Non-published results).
Potential vaccination coverage appears to be better in pneumonia than in other kinds of INV (Table 1). However, the number of isolates from meningitis, arthritis, peritonitis and cellulitis was not sufficient to draw valid conclusions.
Table 4. Potential coverage of three conjugated vaccines in Argentinean pediatric patients with acute otitis media (AOM) caused by S. pneumoniae, H. influenzae or both

|1|PCV7, 7-valent pneumococcal conjugated vaccine; (2)PCV10, 10-valent pneumococcal conjugated vaccine; (3)PCV 13, 13-valent pneumococcal conjugated vaccine; (4)statistically significant differences between PCV7 and PCV10, PCV7 and PCV13, and PCV10 and PCV13 (p < 0.0001, 95 % confidence interval) (Chi-square test, Graph pad Prism, version 5.03).
We would have to take into account that partial coverage of the cross-reactive serotype 6A, but not 19A, would be guaranteed with all conjugated vaccines containing the 6B and the 19F components, as was determined by the opsonophagocytic activity response (7). Considering this possibility, we may speculate that the potential coverage of PCV7 and PCV10 vaccines could increase by 1.8 % for AOM and 3.4 % for INV.
In children younger than 2 years old, the most frequent serotypes from AOM were quite similar to the whole population, but in INV we found differences diminishing the potential coverage of PCV13 from 73 to 65.9 %.
Serotypes 6A, 6B, 9V, 14, 19A, 19F, and 23 F are considered to be the most resistant to antibiotics (2). In our study, 6A, 9V, 14, 33F and 19A were strongly associated with non-susceptibility to penicillin. While 9V and 14 are targets of all three vaccines listed in Table 2, serotypes 6A and 19A are only included in the 13-valent vaccine. As PCV7 was not widely used in our country, the presence of 19A as one of the prevalent serotypes both in AOM and in INV is a matter of real concern because it was involved in severe cases of meningitis, mastoiditis and terapeutic failures in AOM (4, 5). Probably, the recent introduction of the 13-valent conjugate vaccine in the national calendar, would prevent the participation of serotype 19A S. pneumoniae both in INV and AOM.
Serotype 19A was not among the seven more frequently detected serotypes in pediatric INV in Argentina between 1993 and 1997. In SIREVA reports an increasing participation of 19A in INV was observed between periods 1994-1999 (2.3 %), 20002005 (3.5 %) and 2006-2009 (4.7 %) (6).
Almost 40 % of AOM episodes would be prevented by PCV10, while less than 20 % would be covered by PCV13. However, differences in preventing INV were crucial for the decision to include the 13-valent vaccine in the national calendar for children less than two years of age in Argentina.
Acknowledgements: The research leading to these results has received funding from the European Community's Seventh Framework Programme under Grant Agreement No. HEALTH-F3-2009-223111. This research was approved by the Ethical Committee and by the Dirección Asociada de Docencia e Investigación of the Hospital de Pediatría "Prof Dr Juan P Garrahan".
Conflicts of interest: nothing to declare.
1. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; 21st Informational Supplement, 2011; M100-S21. Wayne, PA, USA. [ Links ]
2. Dagan R. Impact of pneumococcal conjugate vaccine on infections caused by antibiotic-resistant Streptococcus pneumoniae. Clin Microbiol Infect 2009; 15 Suppl 3: 1620. [ Links ]
3. Hausdorff WP, Feikin DR, Klugman KP. Epidemiological differences among pneumococcal serotypes. Lancet Infect Dis 2005; 5: 83-93. [ Links ]
4. Moore MR, Gertz RE Jr, Woodbury RL, Barkocy-Gallagher GA, Schaffner W, Lexau C, Gershman K, Reingold A, Farley M, Harrison LH, Hadler JL, Bennett NM, Thomas AR, McGee L, Pilishvili T, Brueggemann AB, Whitney CG, Jorgensen JH, Beall B. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J Infect Dis 2008; 197: 1016-27. [ Links ]
5. Ongkasuwan J, Valdez TA, Hulten KG, Mason EO Jr, Kaplan SL. Pneumococcal mastoiditis in children and the emergence of multidrug-resistant serotype 19A isolates. Pediatrics 2008; 122: 34-9. [ Links ]
6. Organización Panamericana de la Salud. Informe regional de SIREVA II, 2009: datos por país y por grupos de edad sobre las características de los aislamientos de Streptococcus pneumoniae, Haemophilus influenzae y Neisseria meningitidis en procesos invasores. OPS, OMS, Washington DC, 2010. [ Links ]
7. Prymula R, Peeters P, Chrobok V, Kriz P, Novakova E, Kaliskova E, Kohl I, Lommel P, Poolman J, Prieels JP, Schuerman L. Pneumococcal capsular polysaccharides conjugated to protein D provide protection against otitis media caused by both Streptococcus pneumoniae and non-typeable Haemophilus influenzae: a randomized double blind efficacy study. Lancet 2006; 367: 740-8 [ Links ]
8. Reijtman V, Gagetti P, Faccone D, Fossati S, Sommerfleck P, Hernández C, Bernáldez P, Lopardo H, Corso A. Genotypes of macrolide-resistant S. pneumoniae isolated from Argentinean pediatric patients with acute otitis media. 8th International Symposium on Pneumococci and Pneumococcal Diseases, 2012, Abstract 124, p. 56, Foz d'Iguaçu, Argentina. [ Links ]
9. Sommerfleck P, Bernáldez P, Reijtman V, Hernández CM, Lopardo H. Otitis media aguda: prevalencia de otopatógenos en pacientes de un hospital público. Acta Otorrinolaringol Esp 2012. Disponible en: http//dx.doi.org/10.1016/j.otorri.2012.06.005. [ Links ]
10. Vesikari T, Wysocki J, Chevallier B, Karvonen A, Czajka H, Arsène JP, Lommel P, Dieussaert I, Schuerman L. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) compared to the licensed 7vCRM vaccine. Pediatr Infect Dis J 2009; 28 (4 Suppl): S66-76. [ Links ]
11. Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, Reingold A, Cieslak PR, Pilishvili T, Jackson D, Facklam RR, Jorgensen JH, Schuchat A. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med 2003; 348: 1737-46. [ Links ]
12. Williams BG, Gouws E, Boschi-Pinto C, Bryce J, Dye C. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis 2002; 2: 25-32. [ Links ]
Recibido: 14/8/2012
Aceptado: 15/1/2013













