Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista argentina de microbiología
versión impresa ISSN 0325-7541
Rev. argent. microbiol. vol.45 no.2 Ciudad Autónoma de Buenos Aires abr./jun. 2013
MICROBIOLOGÍA BÁSICA
Enhancing adherence of Arcobacter butzleri after serial intraperitoneal passages in mice
Incremento de la adherencia de Arcobacter butzleri por sucesivos pasajes intraperitoneales en ratón
Heriberto Fernández*, Sandra Flores, María P. Villanueva, Gustavo Medina, and Macarena Carrizo
Instituto de Microbiología Clínica, Facultad de Medicina, Universidad Austral de Chile, Valdivia, Chile
*Corresponding author.
E-mail address: hfernand@uach. cl (H. Fernández).
Received 18 September 2012; accepted 23 April 2013
Abstract
We investigated the possibility of enhancing the adherence capacity of four low-adherent Arcobacter butzleri strains after serial intraperitoneal passage (i.p.) in mice. All the strains enhanced their adherence capacity after the first passage, increasing their adhesion rates after each passage. These results suggest that i.p. passage enhances the expression of adherence in A. butzleri strains.
Keywords: Arcobacter butzleri; Virulence; Adhesion; Intraperitoneal passage.
Resumen
Se investigó la posibilidad de incrementar la capacidad de adherencia de cuatro cepas de Arcobacter butzleri de baja adherencia por sucesivos pasajes por peritoneo de ratón. Todas las cepas aumentaron su capacidad de adherencia después del primer pasaje, e incrementaron las tasas de adherencia después de cada pasaje. Estos resultados sugieren que es posible incrementar la expresión de la adherencia en A. butzleri por pasajes intraperitoneales sucesivos en ratón.
Palabras clave: Arcobacter butzleri; Virulencia; Adherencia; Pasaje intraperitoneal.
The genus Arcobacter (Latin: arcus, arch, and Greek: bacter, bacteria) was proposed in 1991 to classify a group of bacteria previously considered to be members of the Campylobacter genus. Molecular studies show that these bacteria have different genotypic characteristics from those of Campylobacter, giving rise to the new genus Arcobacter3. This group is composed of spiral curved, or “S”-shaped, Gram-negative bacilli of 0.2 to 0.6 μm diameters and 3 μm length. They have one or two flagella and grow at temperatures ranging from 15 to 37 ºC, although the optimal growth temperature is 30 ºC. The primary isolation of these bacteria can be achieved under microaerobic conditions, but when using subsequent cultures, they can grow in aerobiosis. Today, the genus Arcobacter is composed of the following species: Arcobacter cryaerophilus, Arcobacter butzleri, Arcobacter skirrowii, Arcobacter nitrofrigilis, Arcobacter cibarius, Arcobacter halophilus, Arcobacter mytili, Arcobacter thereius, Arcobacter marinus, Arcobacter trophiarum, Arcobacter defl uvii, Arcobacter molluscorum and Arcobacter ellisii; however, only the first three have been isolated from human and animal samples3,6,8.
Arcobacter butzleri is an emerging pathogen that has been associated with abortion and enteritis in animals, as well as with diarrhea and bacteremia in adults and children. Currently, A. butzleri is considered the most common species of the genus in environmental water, food and clinical samples, being ranked as the fourth most common campylobacter organism isolated from human feces3,6. Recently, A. butzleri has been considered a serious hazard to human health by the International Commission on Microbiological Specification for Foods3.
The presence of nine putative virulence genes has been reported in A. butzleri ATCC49616 strain and in several human and animal strains by Miller et al.13 and Douidah et al.4, respectively. Some of them, such as cadF (fibronectin binding protein gene), cj1349 (fibronectin binding protein gene), flaA (flagellin A protein gene) and hecA (hemagglutinin gene) share similarities with the virulence genes of Campylobacter jejuni and other bacteria to which they have been associated in adherence4,13.
Adhesive capacity to different epithelial cell lines is a bacterial characteristic associated to pathogenicity, which has been described in A. butzleri by several authors2,6,11.
However, there is no information about changes in the pathogenicity of A. butzleri after animal passages as it occurs with C. jejuni. In fact, several other biological models, such as intraperitoneal animal passage5, intragastric passage in chicks9 and chick embryo passage1,7 have all been used to either enhance or restore virulence capacities and culturability in Campylobacter strains.
The aim of this study was to investigate the possibility of enhancing adhesive properties in weak-adherent A. butzleri strains through intraperitoneal passage (i.p.) in mice. Four strains of A. butzleri from the collection owned by our Institute, originally isolated from river water (three strains) and from chicken liver for human consumption (one strain) were studied. These strains were previously characterized as weakly-adherent to HEp-2 cells by means of the protocol currently being used in our laboratory6. In brief, four Leighton tubes containing a coverslip with the HEp-2 cell monolayers were used for each strain. They were inoculated with 1 ml of the bacterial suspensions (106 CFU) prepared in the same medium that had been used for growing HEp-2 cells [RPMI medium (Gibco) supplemented with 10% fetal calf serum (Gibco)] and incubated at 37 ºC during 3 h, under 5% CO2. Afterwards, coverslips were washed four times with phosphate-buffered saline pH 7.4, fixed with methanol, stained with 10% Giemsa stain and examined under light microscopy (1000 X).
In order to assess induction of adhesive capacity, each strain was subjected to fi ve serial i.p. passages in Rockefeller (3-6 weeks age) mice, being the original strain considered as passage zero. Inocula were prepared in Brucella broth (BB) (Difco) and each animal received 1 ml of a bacterial suspension containing 2 x 109 CFU/ml. After 24 h, mice were euthanized with CO2 and their peritoneal contents were aseptically removed and seeded on blood agar plates that were incubated for 48 h at 30 °C in a microaerobic atmosphere. The adhesive capacity of each of the resulting pure cultures of the isogenic strain was determined by inoculating HEp-2 cell cultures following the above described method. The adhesion rate (number of cells showing adhered bacteria/total cells examined × 100) and the number of bacteria observed in each HEp-2 cell were determined by counting at least 200 cells.
The average number of adhering bacteria was estimated in a minimum of 35 HEp-2 cells with adhered bacteria. All the tests were carried out twice and in duplicate.
The expression of flaA gene in strains AC11 and HP12 was determined by means of the semi-quantitative RT-PCR (sqRTPCR) technique described by Niehus et al.14, using the following flaA primer sequences: sense 5’-CAGTTGCACCAGCTGACATT- 3’ and antisense 5’-TGCAAGAACTGCAAAAGGTG-3’ (amplicon size 158 pb). The rpoB (housekeeping gen) primer sequences used were: sense 5’-CAACTCTTTCAACACCATTAACAA- 3’ and antisense 5’-AGGTAGCGAAGTTGGTAAACCT-3’ (amplicon size 230 bp). First, cDNA was synthesized from total RNA by TaqMan® (Roche) Reverse Transcription Reagents according to the manufacturer’s instructions. Then, RT-PCR reaction was performed using KOD Hot Start DNA Polymerase Novagen®, according to the manufacturer’s instructions. Products from sqRT-PCR were separated and visualized by electrophoresis in agarose gels stained with ethidium bromide. Images of RT-PCR ethidium bromide-stained agarose gels were taken and band quantification was performed with the ImageJ gel analysis free software to determine pixel intensity. Data were normalized with regard to rpoB pixel intensity and expressed as mean ± standard error of the mean (SEM). Semi-quantitative measurements of RNAs were expressed as arbitrary units (AU). Statistical significance was verified by two-way ANOVA - Dunnett’s Multiple Comparison Test. A value of p < 0.05 was considered statistically significant. Data analysis was done with GraphPad Prism5 software.
In order to establish isogenicity, the original strains and the isolates obtained after each i.p. were genotyped using the enterobacterial repetitive intergenic consensus PCR (ERIC-PCR) technique according to Houf et al.12. All the isolates obtained after each i.p. passage showed the same electrophoretic pattern displayed by their respective original strains. As an example, Figure 1 shows the ERIC-PCR electrophoretic patterns of strains AC11 and HP 12.
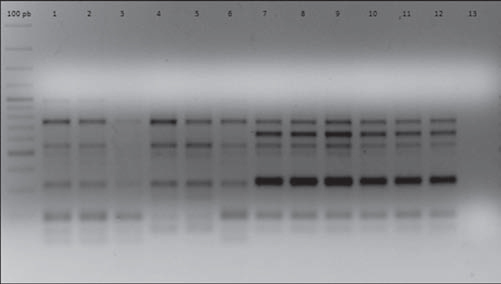
Figure 1 ERIC-PCR electrophoretic patterns of strains AC11 and HP12 and their isogenic strains derived after five successive i. p. passages. Lines 1, 2, 3, 4, 5, 6: strain AC11 P0, P1, P2, P3, P4, P5, respectively. Lines 7, 8, 9, 10, 11, 12: strain HP12 P0, P1, P2, P3, P4, P5, respectively. Line 13: negative control.
As shown in Table 1, In order to establish isogenicity, the original strains and the isolates obtained after each i.p. were genotyped using the enterobacterial repetitive intergenic consensus PCR (ERIC-PCR) technique according to Houf et al.12. All the isolates obtained after each i.p. passage showed the same electrophoretic pattern displayed by their respective original strains. As an example, Figure 1 shows the ERIC-PCR electrophoretic patterns of strains AC11 and HP 12.
Table 1 Enhancement of the adhesive capacity of Arcobacter butzleri through intraperitoneal passages in mice
| Strain | Origin | Passage number | Adherence rate (%)a | Number of adherent bacteria/cellb |
| AC11 | River water | 0 | 46. 0 ± 0. 58 | 1. 53 ± 1. 03 |
|
|
| 1 | 51. 0 ± 1. 53 | 1. 91 ± 1. 01 |
|
|
| 2 | 65. 0 ± 1. 52 | 2. 12 ± 1. 34 |
|
|
| 3 | 68. 0 ± 1. 53 | 2. 41 ± 1. 42 |
|
|
| 4 | 67. 0 ± 1. 00 | 2. 30 ± 1. 45 |
|
|
| 5 | 83. 0 ± 2. 87 | 3. 98 ± 2. 79 |
| AP5 | River water | 0 | 44. 5 ± 0. 86 | 1. 49 ± 0. 93 |
|
|
| 1 | 51. 0 ± 4. 58 | 2. 66 ± 1. 32 |
|
|
| 2 | 62. 5 ± 1. 50 | 1. 87 ± 1. 33 |
|
|
| 3 | 68. 0 ± 1. 00 | 1. 65 ± 0. 96 |
|
|
| 4 | 66. 5 ± 1. 00 | 2. 26 ± 1. 51 |
|
|
| 5 | 66. 0 ± 0. 76 | 2. 77 ± 1. 30 |
| AA10 | River water | 0 | 31. 0 ± 1. 73 | 0. 96 ± 0. 61 |
|
|
| 1 | 46. 5 ± 1. 32 | 1. 72 ± 1. 04 |
|
|
| 2 | 59. 5 ± 2. 00 | 1. 74 ± 1. 01 |
|
|
| 3 | 58. 5 ± 3. 46 | 2. 14 ± 1. 62 |
|
|
| 4 | 73. 5 ± 4. 58 | 3. 55 ± 1. 18 |
|
|
| 5 | 80. 5 ± 1. 80 | 4. 09 ± 3. 42 |
| HP12 | Chicken liver | 0 | 45. 0 ± 1. 32 | 1. 49 ± 0. 93 |
|
|
| 1 | 53. 0 ± 0. 57 | 2. 66 ± 1. 32 |
|
|
| 2 | 47. 5 ± 1. 50 | 1. 87 ± 1. 33 |
|
|
| 3 | 53. 0 ± 1. 52 | 1. 65 ± 0. 96 |
|
|
| 4 | 59. 0 ± 0. 76 | 2. 26 ± 1. 51 |
|
|
| 5 | 60. 0 ± 2. 64 | 2. 57 ± 1. 10 |
ap < 0. 05 when comparing the results of each passage with that of passage 0, except passage 2 of strain AA10; bp > 0. 05 when comparing the results of each passage with that of passage 0, except passage 5 of strain AA10.
Strain HP12, isolated from chicken liver, showed the lowest number of adhering bacteria per cell (1.49 ± 0.93 and 2.57 ± 1.10) after the first and the fifth passages, whereas strains AC11 and AA10, isolated from river water, showed the highest numbers of adhered bacteria per cell after the fifth passage (3.98 ± 2.79 and 4.09 ± 3.42, respectively). However, numbers of adherent bacteria increased after each passage, with the exception of passage 5 of strain AA10. No differences (Student´s t-test) were observed when comparing the increase of adherent bacteria in each passage with the number of adherent bacteria observed in passages 0 of each strain.
In general, despite the description of nine putative virulence genes4,5, little is known about the pathogenic mechanisms or potential virulence factors of Arcobacter spp. and how they could be expressed in both in vitro and in vivo models. In this study, we used the mouse i. p. passage model as a biological support, in an attempt to increase the adhesive capacity in A. butzleri strains that had previously shown low adherence rates. As shown in Table 1, all four strains enhanced their adhesive capacity after several consecutive passages, with the adhesion rates and the number of adherent bacteria increasing from the first to the fifth passage, with very few exceptions.
Several biological models have been used to enhance virulence in different bacteria. Fernández et al.5 reported that C. jejuni and Campylobacter coli strains enhanced their enterotoxigenic and invasive capacities after i.p. passages in mice. Hänel et al.9 demonstrated that upon passage through the chicken gut, a C. jejuni strain enhanced its adherence to eukaryotic cells.
Despite the existence of several studies on adhesion, invasion and toxigenic capacities, the pathogenicity and virulence mechanisms of A. butzleri are still poorly understood3. On the other hand, there are few experimental trials exploring the pathogenic properties of Arcobacter in animal models.
The first in vivo study inoculating Arcobacter strains intravenously or intraperitoneally in rodents was unsuccessful. No clinical symptoms were observed and no internal lesions were detected after postmortem examination10. However, the invasion and colonization capacity of A. butzleri has been demonstrated in White turkeys by Wesley and Baetz15, who reported that adapting A. butzleri to poultry via serial passage may increase the ability of the organism to colonize outbred birds.
The mouse i.p. passage model used in this study enhanced the adherence capacity of A. butzleri strains after the first passage, with an increase in adhesion rates after each passage giving a new insight into the pathogenicity mechanisms of this bacterium. These results show that this model is suitable for inducing expression of adhesion capacity in A. butzleri, having the additional advantage that the isogenic variants can be isolated in pure cultures on non-selective media due to the absence of contaminant microbiota in the i.p. cavity.
In an attempt to have an approximation to the molecular basis that could explain the increase of the adhesion capacity in the strains studied, we analyzed the expression of the flaAgene in the original strains AC11 and HP12 and their isogenic strains derived from i. p. passages 1, 3 and 5. As shown in Figure 2, the expression of the flaA gene of strain AC11 was significantly increased.
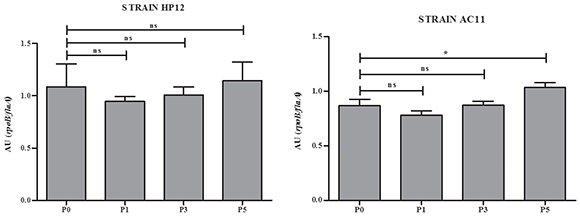
Figure 2 Relative semi-quantification of flaA gene expression in strains HP12 and AC11 and their isogenic strains derived after i. p. passages P1, P3 and P5. AU: arbitrary units; ns: non-significant; P0: Passage 0; P1: Passage 1; P3: Passage 3; P5: Passage 5.
*p < 0. 05
Miller et al.13 and Douidah et al.4 reported nine putative virulence genes in A. butzleri that had been previously reported in C. jejuni. It is known that the fl aA gene is associated with adherence capacity in C. jejuni and A. butzleri4,13. The increased expression of the flaA gene after the fifth i.p. passage in strain AC11, although still not conclusive, suggests that changes in the gene expression of A. butzleri can occur through successive passages. However, further studies are necessary to elucidate the molecular basis of this phenomenon and also to determine if A. butzleri can carry its own virulence genes, other than those reported by Miller et al.13 and Douidah et al.4.
Conflict of interest
The authors declare that they have no conflicts of interest.
Acknowledgments
The authors would like to thank Dr. Hans G. Richter (Departmento de Anatomía, Histología y Patología, Universidad Austral de Chile) for his advice and critical review. This work was financially supported by grant FONDECYT 1110202.
1. Baffone W, Casaroli A, Citterio B, Pierfelici L, Campana R, Vittoria E, Guaglianone E, Donelli G.Campylobacter jejuni loss of culturability in aqueous microcosms and ability to resuscitate in a mouse model. Int J Food Microbiol. 2006;107:83-91. [ Links ]
2. Bücker R, Troeger H, Kleer J, Fromm M Schulzke JD.Arcobacter butzleri indices barrier dysfunction in intestinal HT-29/B6 cells. J Infect Dis. 2009; 200:756-64. [ Links ]
3. Collado L, Figueras MJ. Taxonomy, epidemiology, and clinical relevance of the genus Arcobacter. Clin Microbiol Rev. 2011;24:1174-92. [ Links ]
4. Douidah L, de Zutter L, Baré J, DeVos P, Vandamme P, Vandenberg O, Van den Abeele AM, Houf K. Occurrence of putative virulence genes in Arcobacter species isolated from humans and animals. J Clin Microbiol. 2012;50:735-41. [ Links ]
5. Fernández H, Vivanco T, Eller G. Expression of invasiveness of Campylobacter jejuni subsp.jejuni after serial intraperitoneal passages in mice. J Vet Med B. 2000;47:635-9. [ Links ]
6. Fernández H, Flores S, Inzunza F.Arcobacter butzleri strains isolated from different sources display adhesive capacity to epithelial cells in vitro. Acta Scient Vet. 2010;38:287-91. [ Links ]
7. Field LH, Underwood JL, Payne SM, Berry L J. Characteristics of an avirulent Campylobacter jejuni strain and its virulence enhanced variants. J Med Microbiol. 1993;38:293-300. [ Links ]
8. Figueras MJ, Levican A, Collado L, Inza MI, Yustes C.Arcobacter ellisii sp. nov. , isolated from mussels. Syst Appl Microbiol. 2011;34:414-8. [ Links ]
9. Hänel I, Borrmann E, Müller J, Müller W, Pauly B, Liebler- Tenorio EM, Schulze F. Genomic and phenotypic changes of Campylobacter jejuni strains after passage of the chicken gut. Vet Microbiol. 2009;136:121-9. [ Links ]
10. Higgins R, Degre R. Isolation of spirillum-like organisms from pig and bovine fetuses. Vet Rec. 1979;104:262-3. [ Links ]
11. Hoa TKH, Lipman LJA, Hendriks HGCJM, Tooten PCJ, Ultee T, Gaastra W. Interaction of Arcobacter spp. with human and porcine intestinal epithelial cells. FEMS Immunol Med Microbiol. 2007;50:51-8. [ Links ]
12. Houf K, de Zutter L,Van Hoof J, Vandamme P. Assessment of the genetic diversity among arcobacters isolated from poultry products by using two PCR-based typing methods. Appl Environ Microbiol. 2002;68:2172-8. [ Links ]
13. Miller WG, Parker CT, Rubenfi eld M, Mendz GL, Wösten MM, Ussery DW, Stolz JF, Binnewies TT, Hallin PF, Wang G, Malek JA, Rogosin A, Stanker LH, Mandrell RE. The complete genome sequence and analysis of the epsilon proteobacterium Arcobacter butzleri. PLoS One. 2007;2:e1358. [ Links ]
14. Niehus E, Gressmann H, Fang Y, Schlapbach R, Dehio M, Dehio C, Stack A, Meyer TF, Suerbaum S, Josenhans C. Genome-wide analysis of transcriptional hierarchy and feedback regulation in the fl agellar system of Helicobacter pylori. Mol Microbiol. 2004;52:947-61. [ Links ]
15. Wesley IV, Baetz AL. Natural and experimental infections of Arcobacter in poultry. Poult Sci. 1999;78:536-45. [ Links ]














