Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista argentina de microbiología
versión impresa ISSN 0325-7541
Rev. argent. microbiol. vol.46 no.2 Ciudad Autónoma de Buenos Aires jun. 2014
ORIGINAL ARTICLE
Seroprevalence and major antigens recognized by sera from Trypanosoma cruzi-infected dogs from Jalisco, México
Seroprevalencia y principales antígenos reconocidos por sueros de perros infectados con Trypanosoma cruzi en el estado de Jalisco, México
Ignacio Martíneza, Alejandro Martínez-Ibarrab, Minerva Arce-Fonsecac, Olivia Rodríguez-Moralesc, Deyanira Pérez-Moralesa, Pedro A. Reyes Lópezd, Bertha Espinozaa*
aDepartamento de Inmunología, Instituto de Investigaciones Biomédicas, Universidad Nacional Autónoma de México, Del. Coyoacán, México City, México
bÁrea de Entomología Médica, Centro Universitario del Sur, Universidad de Guadalajara, Ciudad Guzmán, Jalisco, México
cDepartamento de Biología Molecular, Instituto Nacional de Cardiología "Ignacio Chávez", México City, México
dLaboratorio de Inmunología Molecular y Proteómica, Dirección de Investigación, Instituto Nacional de Cardiología "Ignacio Chávez", México City, México
Received 4 February 2014; accepted 5 May 2014
* Corresponding autor.
E-mail addresses: besgu@biomedicas.unam.mx, besgu@servidor.unam.mx (B. Espinoza).
Abstract
Chagas disease is a major endemic disease caused by the protozoan parasite Trypanosoma cruzi. This parasitic disease is widely distributed throughout Latin America, affecting 10 million people. There are also reports of canine infection in the southern part of the United States. Dogs are considered the predominant domestic reservoir for T. cruzi in many areas of endemicity. In México, dog infection by this parasite has been poorly studied. In this work 209 dogs from six villages in Jalisco, México, were assessed to detect anti-T. cruzi antibodies by ELISA and Western blot. Seventeen (17) seropositive dogs (8.1 %) were detected by both tests, representing a seropositive value similar to that found in some southern states of México where the infection is present. No statistical differences were observed concerning the age and sex of infected and non-infected dogs. The major antigens recognized by positive sera were 26, 32, 66 and 80 kDa. These proteins are candidates to develop a specific diagnostic method for canine Chagas. No antibodies against HSP16 protein were found in T. cruzi seropositive sera. This is the first report of canine serology of Chagas disease in this central part of México. This report will contribute to the knowledge of the infection status of domestic reservoirs in the state of Jalisco, México.
Keywords
Canine Chagas; Infected dogs; Trypanosoma cruzi.
© 2014 Asociación Argentina de Microbiología. Published by Elsevier España, S.L. All rights reserved.
Resumen
El mal de Chagas es una enfermedad endémica causada por el parásito protozoario Trypanosoma cruzi. Este padecimiento está ampliamente distribuido en América, donde afecta a alrededor de 10 millones de personas. También existen comunicaciones de la infección canina desde el sur de los Estados Unidos hasta países de Sudamérica. Los perros son considerados los principales reservorios domésticos de T. cruzi en muchas áreas endémicas. En México, la infección canina ha sido estudiada escasamente. En el presente trabajo se evaluó mediante ELISA y Western blot la presencia de anticuerpos anti-T. cruzi en el suero de 209 perros de seis localidades del estado de Jalisco, México. Se encontraron 17 perros seropositivos (8,1 %) a ambas pruebas. No se observaron diferencias de significación estadística en la edad o el sexo de los perros infectados comparados con los no infectados. Los principales antígenos reconocidos por los sueros positivos fueron de 26, 32, 66 y 80 kDa. Estas proteínas son candidatos para desarrollar un método de diagnóstico específico para Chagas canino. No se encontraron anticuerpos contra la proteína HSP16 en los sueros positivos anti-T. cruzi. Este es el primer informe de serología canina en la región central de México y contribuirá al conocimiento de la infección en reservorios domésticos de Jalisco, México.
Palabras clave
Chagas canino; Perros infectados; Trypanosoma cruzi.
© 2014 Asociación Argentina de Microbiología. Publicado por Elsevier España, S.L. Todos los derechos reservados.
Introduction
Chagas disease is a major public health concern and veterinary pathogen in American countries, where 10 million infected inhabitants have been reported25. The infection is also present in wild mammals and domestic dogs in the United States3. Trypanosoma cruzi, the etiologic agent of Chagas disease, has a life-cycle that involves several Triatomine species as vectors8. The infestation of houses and peridomestic areas is considered to be a major risk factor for Chagas disease transmission7. T. cruzi can infect humans and cause megacolon, megaesophagus and several cardiomyopathies, including cardiomegaly, arrhythmias and conduction disorders25. Domestic mammals, especially dogs, have been found to be naturally infected with T. cruzi, and their presence in dwellings has been considered to be a risk factor to humans because dogs can be a domestic reservoir of T. cruzi and a feed source for insect vectors28. Infected dogs have been reported in several American countries, including the United States, in which the seroprevalence of specific anti-T. cruzi antibodies has also been reported4,6,19,23. However, in México, where human infection is present in extended areas, dog infection has been poorly studied. The states of México, Puebla, Chiapas, Morelos, Campeche and Yucatán are the only ones (6 out of 32) where dogs infected with T. cruzi have been reported1,10,12,13,26. Jalisco state, in central México, is an endemic area where seven Triatomine species infected with T. cruzi have been found in houses and peridomestic areas and where human T. cruzi infections have been reported since 196715,16. Several serologic studies of blood banks and the general population have detected human infection, and the presence of chronic chagasic cardiomyopathy (CCC) and digestive disorders have also been reported9,15,27.
However, to date, T. cruzi seroprevalence in domestic reservoirs such as dogs has not been reported.
The aim of the present work was to determine the seroprevalence of natural infection in domestic dogs from several villages in southeast Jalisco, México, where infected vectors have been previously reported. The antigens recognized by sera of these dogs are also reported. Furthermore, antibodies against recombinant protein HSP16 were evaluated, since a homologous antigenic protein has been previously reported in canine leishmaniasis18.
Materials and methods
Study area and sera collection
Six villages (Teocuitatlán, Puerta de Cítala, Cítala, San José de Gracia, Tierra Blanca and Lázaro Cárdenas) in the area of Teocuitatlán de Corona, Jalisco, México, were selected because the insect vectors of Chagas disease have previously tested positive for T. cruzi in this area. Teocuitatlán de Corona is located in the southeast of Jalisco (20º01'30" to 20º12'30" N and 103º11'20" to 103º30'00" W) between Guadalajara city and Ciudad Guzmán, which are both major towns of Jalisco state. A blood sample from the saphenous vein of 209 domestic dogs living in these villages was collected on filter paper with the consent and in the presence of their owners. Age and sex of the dogs were recorded. None of the dogs had lesions consistent with leishmaniasis. The blood on the filter paper was dried, and subsequently eluted in laboratory as described previously11. Briefly, the filter paper with dry blood was cut, and the sample was eluted in the presence of PBS (884 ?l/cm2) overnight at 4ºC. A total of 25 ?l of eluted blood was considered to be equivalent to 1 ?l of concentrated serum. Eluted samples were stored at -20ºC until use.
Control sera
Anti-T. cruzi positive sera were obtained from dogs that were inoculated intraperitoneally with 5x104 or 1-2x105 T. cruzi metacyclic trypomastigotes of the Ninoa strain (MHOM/MX/1994/Ninoa) obtained from the feces of infected vectors. Sera were collected 8 months post-inoculation. Animal handling followed the established guidelines of the International Guiding Principles for Biomedical Research involving Animals and the Norma Oficial Mexicana (NOM-0062-ZOO 1999). Technical Specifications for the Care and Use of Laboratory Animals and the experimental protocol were approved by the Bioethics Committee of the Instituto Nacional de Cardiología "Ignacio Chávez". Negative control sera were obtained from the venous blood of four healthy domestic dogs from México City without past exposure to T. cruzi.
Antibodies anti-T. cruzi determination by ELISA and Western blot
Total proteins were extracted by sonicating the Querétaro strain of T. cruzi (TBAR/MX/0000/Querétaro) in the presence of protease inhibitors. Aliquots of 1 ?g/well were added to 96-well plates (costar 3590) following a previously described protocol24. ELISA was performed using canine serum or eluted blood diluted 1:500, and the secondary anti-IgG dog antibody (ICN/Cappel, 55332) was diluted 1:10,000. The optical density (O.D.) was read from the microtiter plates with an ELISA reader (Bio-Rad Model 450) at 490 nm. Each test was carried out in duplicate. The ELISA cut-off was established by calculating the OD average of negative controls plus 2.5 standard deviations. All samples with an O.D. value higher than the cut-off value were considered to be positive. Western blotting (Wb) was performed using nitrocellulose membranes containing 3.5 ?g/strip of protein from the total extract according to a previously described protocol24. Dog sera or eluted blood were diluted 1:500, and the secondary antibody was used at 1:12,500. Antigen registration was performed with a Gel Logic 2000 image capture system and analyzed by Kodak MI software version 3.1 (Kodak, USA).
Sera reactivity to recombinant sHSP16 protein
Recombinant T. cruzi small heat-shock protein 16 (HSP16) has been previously cloned and characterized21. The recombinant protein was run on an SDS-PAGE gel and transferred to a nitrocellulose membrane. Strips containing 40 ng of protein were used for Wb as described above. ELISA of sHSP16 was performed using 150 ng/well of recombinant HSP16 in 96-well plates following the protocol described above.
Statistical analysis
Univariate analysis to determine the association between the age and sex of the dogs and T. cruzi seropositivity was performed via a two-tailed Fisher's exact test using GraphPad QuickCalcs free statistical calculators, (La Jolla, California). Available at: www.graphpad.com/quickcalcs/ contingency1.cfm. Accessed on Aug 10, 2013.
Results
Two hundred and nine dogs from six villages in the locality of Teocuitatlán de Corona, Jalisco, México, were evaluated for anti-T. cruzi antibodies by ELISA and Wb. Both tests showed seropositivity in 17 of them. One sample from Teocuitatlán was ELISA positive but gave a weak band in Wb and was considered a discordant sample. Seroprevalence by village ranged from 7.7 to 15.4 % (Table 1). Mean seroprevalence was 8.1 %. There was no a statistical significant difference with respect to the sex of the dogs (p=0.214). Seropositive dogs ranged in age from 2 to 72 months old. Moreover, there was no statistical difference in relation to the age of the dogs (p= 0.6370).
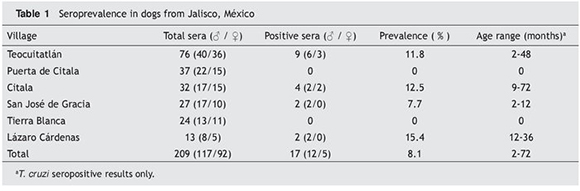
Heterogeneity in the ELISA OD values was observed (Fig. 1A). Differences were also found in the antigens recognized by the eluted dog blood (Fig. 1B), which ranged in size from 26 to over 250 kDa. Seropositive samples from the San José de Gracia and Lázaro Cárdenas communities recognized few antigens. In contrast, samples from three of the four seropositive dogs from the Cítala community recognized multiple antigens of T. cruzi. There was no association between the sex and age of the dogs and the recognized protein banding patterns.

Figure 1. Serology of infected dogs. A) ELISA was performed on control and Jalisco sera. Mean values are indicated by a line. B) Western blot showing the antigens recognized by dog sera from seropositive controls (Pos), seronegative controls (Neg), representative seronegative sera from Teocuitatlán (NT) and seropositive dogs from San José de Gracia (SJG), Lázaro Cárdenas (LC), Teocuitatlán (the fourth sample was ELISA positive but presented a very weak band and was considered discordant) and Cítala, Jalisco.
The major antigens recognized by eluted blood from the Jalisco dogs were 66 kDa (94 %), 32 kDa (61 %), 26 kDa (55 %) and 80 kDa (50 %). These antigens were also recognized by positive control sera, but in the latter case two other antigens (35 and 72 kDa) were also recognized (Fig. 1B).
Antibodies against HSP16 were evaluated by ELISA and Wb in the eluted dog blood from Jalisco. Differences between the control and infected samples were not found (data not shown).
Discussion
Dog infection with T. cruzi is an important veterinary health concern in many countries of South America and more recently in the United States. Infected vectors have been reported in Texas military kennels, where some dogs showed clinical signs compatible with T. cruzi infection17. Parasites from infected dogs in some states have also been isolated20. In México, including Jalisco and Oaxaca states, dogs have been reported to be frequent blood source for Triatomine insects2,28. Dogs have been considered to be risk factors for Chagas disease when they are infected with T. cruzi and they live in close contact with humans6,13. In this work it was found that 17 of 209 dogs (8.1%) were seropositive. This seroprevalence value is similar to that reported in Morelos and higher than that reported in Chiapas, which are both Chagas disease-endemic states in México12,13. Seropositivity in young dogs (2 months old) can be possibly explained as follows: firstly, the vector was living in the house and the puppies were infected in the first months of life; secondly, the mothers were infected during pregnancy (connatal transmission of T. cruzi could occur, as has been previously demonstrated in a canine model)22, and thirdly, anti-T. cruzi antibodies were transferred to the puppy from the infected mother. To clarify this point, more research must be done in this field.
Heterogeneity in ELISA OD values was observed (Fig. 1A). Differences were also detected in the antigens recognized by eluted dog blood (Fig. 1B), which ranged in size from 26 to over 250 kDa. These differences may be attributable to different infective strains, differences in the immune response of each dog or differences in the time of infection. It will be important to establish the identity of the major antigens recognized (26, 32, 66 and 80 kDa), in order to use them for a more specific diagnosis. An 80 kDa antigen was previously recognized by sera of seropositive dogs from Campeche, México1. These results are similar to those in a previous work reporting the presence of antibodies against an 80 kDa antigen that belongs to the transferrin family of T. cruzi in dogs experimentally infected with the parasite5. It will be interesting to test if the antigen reported in this work is the same.
Additionally, it has been previously reported that some small heat shock proteins (HSPs), like Leishmania HSP20 and Echinococcus HSP20, can be antigens recognized during natural infections in dogs14,18. The homologue molecule (HSP16) of T. cruzi has been characterized by our laboratory21. No difference was found in the recognition of the protein by using sera from infected or control dogs. Differences in the antigenicity of T. cruzi HSP16 and other HSPs may be due to low identity between them and to the fact that some antigenic epitopes are likely missing in the T. cruzi molecule. Differences in the amino acid sequence may also involve differences in glycosylation patterns that are important for the antigenicity of the molecule.
This is the first report of canine serology of Chagas disease in this central-western region of México. This report will contribute to the knowledge of the infection status of domestic reservoirs in the state of Jalisco, México, and therefore, to a better understanding of Chagas disease epidemiology.
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that no patient data appear in this article.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
Funding for this study was provided by DGAPA-UNAM, grant number IN206512; Oficina de Colaboración Interinstitucional CTIC-UNAM, grant number PZ-353; Project CUSURUDG-2007-06; Instituto de Ciencia y Tecnología del Distrito Federal, grant number PICSA10-130; grant from Fundación Miguel Alemán to Bertha Espinoza.
1. Balán LU, Yerbes IM, Piña MA, Balmes J, Pascual A, Hernández O, López R, Monteón V. Higher seroprevalence of Trypanosoma cruzi infection in dogs than in humans in an urban area of Campeche, México. Vector-Borne Zoonotic Dis. 2011;11:483- 4. [ Links ]
2. Bosseno MF, García LS, Baunaure F, Gastelúm EM, Gutiérrez MS, Kasten FL, Dumonteil E, Breniére SF. Identification in triatomine vectors of feeding sources and Trypanosoma cruzi variants by heteroduplex assay and a multiplex miniexon polymerase chain reaction. Am J Trop Med Hyg. 2006;74:303-5. [ Links ]
3. Charles RA, Kjos S, Ellis AE, Barnes JC, Yabsley MJ. Southern plains woodrats (Neotoma micropus) from southern Texas are important reservoirs of two genotypes of Trypanosoma cruzi and host of a putative novel Trypanosoma species. Vector- Borne Zoonotic Dis. 2013;13:22-30. [ Links ]
4. Cohen JE. Domestic dogs and cats as sources of Trypanosoma cruzi infection in rural northwestern Argentina. Parasitology. 2007;134:69-82. [ Links ]
5. Corral RS, Orn A, Freilij HL, Bergman T, Grinstein S. Purification and characterization of an 80-kilodalton Trypanosoma cruzi urinary antigen. J Clin Microbiol. 1989;27:145-51. [ Links ]
6. Crisante G, Rojas A, Teixeira MM, Añez N. Infected dogs as a risk factor in the transmission of human Trypanosoma cruzi infection in western Venezuela. Acta Trop. 2006;98:247-54. [ Links ]
7. Esch KJ, Petersen CA. Transmission and epidemiology of zoonotic protozoal diseases of companion animals. Clin Microbiol Rev. 2013;26:58-85. [ Links ]
8. Espinoza B, Martínez-Ibarra JA, Villalobos G, de la Torre P, Laclette JP, Martínez F. Genetic variation of North American Triatomines (Insecta: Hemiptera: Reduviidae): Initial divergence between species and populations of Chagas disease vectors. Am J Trop Med Hyg. 2013;88:275-84. [ Links ]
9. Espinoza B, Vera-Cruz JM, González H, Ortega E, Hernández R. Genotype and virulence correlation within Mexican stocks of Trypanosoma cruzi isolated from patients. Acta Trop. 1998;70:63-72. [ Links ]
10. Estrada-Franco JG, BhatiaV, Diaz-Albiter H, Ochoa-García L, Barbabosa A, Vázquez-Chagoyán JC, Martínez-Perez MA, Guzmán-Bracho C, Garg N. Human Trypanosoma cruzi infection and seropositivity in dogs, México. Emerg Infect Dis. 2006;12:624-30. [ Links ]
11. Fenollar F, Raoult D. Diagnosis of rickettsial diseases using samples dried on blotting paper. Clin Diagn Lab Immunol. 1999;6:483-8. [ Links ]
12. García-Vázquez R, Cruz R, Miranda-Miranda R, Domínguez- Márquez A. A serological survey of Trypanosoma cruzi infection in dogs of two urban areas of México. Prev Vet Med. 1995;25: 1-6. [ Links ]
13. Gúrtler RE, Cecerre MC, Lauricella MA, Cardinal MV, Kitron U, Jiménez-Coello M, Ortega-Pacheco A, Guzmán-Marin E, Guiris- Andrade DM, Martínez-Figueroa L, Acosta-Viana KY. Stray dogs as reservoirs of the zoonotic agents Leptospira interrogans, Trypanosoma cruzi and Aspergillus spp. in an urban area of Chiapas in southern México. Vector-Borne Zoonotic Dis. 2010;10:135-41. [ Links ]
14. Kouguchi H, Matsumoto J, Katoh Y, Suzuki T, Oku Y, Yagi K. Echinococcus multilocularis: two-dimensional Western blotting method for the identification and expression analysis of immunogenic proteins in infected dogs. Exp Parasitol. 2010;124:238-43. [ Links ]
15. Lozano-Kasten F, Magallón-Gastélum E, Soto-Gutiérrez M, Kasten-Monges M, Bosseno MF, d'Etude I, Breniére SF. Epidemiologic knowledge and current situation of Chagas disease in the state of Jalisco, México. Salud Pública Mex. 2008;50:508-15. [ Links ]
16. Martínez-Ibarra JA, Grant-Guille Y, Morales-Corona N, Haro- Rodriguez S, Ventura-Rodriguez LV, Nogueda-Torres B, Bustos- Saldaña R. Importance of species of Triatominae (Heteroptera: Reduviidae) in risk of transmission of Trypanosoma cruzi in western México. J Med Entomol. 2008;45:476-82. [ Links ]
17. McPhatter L, Roachell W, Mahmood F, Haro-Rodriguez S, Ventura-Rodriguez LV, Nogueda-Torres B, Bustos-Saldaña R. Vector surveillance to determine species composition and occurrence of Trypanosoma cruzi at three military installations in San Antonio, Texas. US Arm Med Dep J. 2012;3:12-21. [ Links ] [ Links ]
18. Montalvo-Alvarez AM, Folgueira C, Carrión J, Monzote- Fidalgo L, Canavate C, Requena JM. The Leishmania HSP20 is antigenic during natural infections, but, as DNA vaccine, it does not protect BALB/c mice against experimental L. amazonensis infection. J Biomed Biotech. 2008. doi:10.1155/2008/695432.
19. Morais AN, Sousa MG, Meireles LR, Kesper N, Umezawa ES. Canine visceral leishmaniasis and Chagas disease among dogs in Araguaína, Tocantins. Rev Bras Parasitol Vet. 2013;22: 225-9. [ Links ]
20. Patel JM, Rosypal AC, Zimmerman KL, Monroe WE, Sriranganathan N, Zajac AM, Yabsley MJ, Lindsay DS. Isolation, mouse pathogenicity, and genotyping of Trypanosoma cruzi from an English Cocker Spaniel from Virginia, USA. Vet Parasitol. 2012:187:394-8. [ Links ]
21. Pérez-Morales D, Ostoa-Saloma P, Espinoza B. Trypanosoma cruzi SHSP16: Characterization of an α-crystallin small heat shock protein. Exp Parasitol. 2009;123:182-9.
22. Rodríguez-Morales O, Ballinas-Verdugo MA, Alejandre-Aguílar R, Reyes PA, Arce-Fonseca M. Trypanosoma cruzi connatal transmission in dogs with Chagas disease: experimental case report. Vector-Borne Zoonotic Dis. 2011;11:1365-70. [ Links ]
23. Rosypal AC, Tripp S, Kinlaw C, Sharma RN, Stone D, Dubey J. Seroprevalence of canine leishmaniasis and american trypanosomiasis in dogs from Grenada, West Indies. J Parasitol. 2010;96:228-9. [ Links ]
24. Sánchez B, Monteón V, Reyes PA, Espinoza B. Standardization of micro-enzyme-linked immunosorbent assay (ELISA) and western blot for detection of Trypanosoma cruzi antibodies using extracts from Mexican strains as antigens. Arch Med Res 2001;32:382-8. [ Links ]
25. Savioli L, Daumerie D. Chagas disease (American trypanosimiasis). In First WHO report on neglected tropical diseases: working to overcome the global impact of neglected tropical diseases. Geneva, Switzerland, World Health Organization; 2010. [ Links ]
26. Sosa-Jurado F, Zumaquero-Ríos JL, Reyes PA, Cruz-García A, Guzmán-Bracho C, Monteón VM. Biotic and abiotic determinants of seroprevalence of antibodies against Trypanosoma cruzi in Palmar de Bravo, Puebla, México. Salud Pública Mex 2004;46:39- 48. [ Links ] In Spanish.
27. Trujillo-Contreras F, Villanueva-Yerenas MA, Soto-Gutiérrez M, Raygoza-Anaya M, Jiménez-Corder A. Serological monitoring of Trypanosoma cruzi infection in individuals studied in 50 municipalities of the State of Jalisco, México in 1987-1994. Rev Soc Bras Med Trop. 2000;33:591-6. [ Links ]
28. Villalobos G, Martínez-Hernández F, de la Torre P, Laclette JP, Espinoza B. Entomological indices, feeding sources, and molecular identification of Triatoma phyllosoma (Hemiptera: Reduviidae) one of the main vectors of Chagas disease in the Istmo de Tehuantepec, Oaxaca, México. Am J Trop Med Hyg. 2011;85:490-7. [ Links ]














