Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista argentina de microbiología
Print version ISSN 0325-7541
Rev. argent. microbiol. vol.46 no.2 Ciudad Autónoma de Buenos Aires June 2014
ORIGINAL ARTICLE
Optimization of laccase production by two strains of Ganoderma lucidum using phenolic and metallic inducers
Optimización de la producción de lacasa por dos cepas de Ganoderma lucidum utilizando inductores fenólicos y metálicos
Francisco Kuhara,*, Leandro Papinuttib
aCentro de Investigación y Extensión Forestal Andino Patagónico (CIEFAP), Esquel, Chubut, Argentina
bLaboratorio de Micología Experimental, Departamento de Biodiversidad y Biología Experimental, Facultad de Ciencias Exactas y Naturales, Ciudad Autónoma de Buenos Aires, Argentina
Recibido el 18 de junio de 2013; aceptado el 30 de abril de 2014
* Corresponding author.
E-mail address: fkuhar@ciefap.org.ar (F. Kuhar).
Abstract
Ganoderma lucidum (Curtis) P. Karst is a white rot fungus that is able to degrade the lignin component in wood. The ability of two strains of this species to produce the ligninolytic enzyme laccase was assessed. After the evaluation of induction with heavy metals and phenolic compounds, it was found that among the tested substances, copper and ferulic acid are the best laccase inducers. It was also observed that the two types of inducers (phenolic and metallic) produce different electrophoretic patterns of laccase activity. Optimized concentrations of inducers were obtained through a factorial design and the thermal stability of optimized supernatants was studied at a wide range of acidic pH. We found that the enzyme is more thermostable at higher pH values.
Keywords
Ganoderma; Laccase; Induction; Optimization; Phenolic compounds; Copper.
© 2014 Asociación Argentina de Microbiología. Published by Elsevier España, S.L. All rights reserved.
Resumen
Ganoderma lucidum (Curtis) P. Karst es un hongo causante de pudrición blanca, capaz de degradar la lignina de la madera y otros sustratos en los que crece. En este trabajo se evaluó la capacidad de dos cepas de esta especie de producir la enzima ligninolítica lacasa. Asimismo, se ensayó la inducción de esta enzima con diferentes compuestos fenólicos e iones metálicos, y se encontró que el ácido ferúlico y el cobre fueron los mejores inductores de la lacasa entre los agentes evaluados. También se encontró que los dos tipos de inductores (fenólicos y metálicos) producen distintos patrones electroforéticos de actividad lacasa. Las concentraciones óptimas de los inductores fueron establecidas mediante un diseño factorial. Se estimó la estabilidad térmica de la lacasa en un amplio rango de pH ácidos, y se comprobó que a pH más altos la enzima es más termoestable.
Palabras clave
Ganoderma; Lacasa; Inducción; Optimización; Compuestos fenólicos; Cobre.
© 2014 Asociación Argentina de Microbiología. Publicado por Elsevier España, S.L. Todos los derechos reservados.
Introduction
Ligninolytic enzyme production by Ganoderma lucidum has been studied in recent decades with special emphasis on laccase activity due to its efficiency as lignin degrader and its low production of Mn peroxidase and lignin peroxidase13,17-19. In other species of this genus, high laccase production in solid as well as in liquid medium has been verified13,16-19. Induction of laccase activity by phenolic compounds has been reported in many genera of fungi and also in G. lucidum5. The induction of this enzyme using metals has been studied in other genera of white rot fungi6 such as copper on Trametes trogii10. To date there are no published reports on the induction of laccase activity by metals in G. lucidum. The thermal stability of fungal laccases is generally higher at acidic pH3, although there have been exceptions1. Stability varies considerably at different temperatures. The half-life at 50 °C ranges from minutes in Botrytis cinnerea to more than 2-3 hours in Agaricus bisporus and Lentinula edodes, to a maximum of 50-70 hours in Trametes sp.2 While laccase enzyme activity disappears immediately at 60 ºC as reported by Baldrian2, the laccase of Melanocarpus albomyces exhibits a half-life of more than 5 h at 60 ºC and hence a very high potential for certain biotechnological applications11,12. The goals of our work are: to combine metallic and phenolic inducers in order to optimize laccase production in a liquid medium and to estimate the thermostability of the optimized supernatant.
Materials and methods
Strains and cultures
Ganoderma lucidum strains E47 (University of Guelph, Canada) and S (cultures and strains collection of CERZOS Bahía Blanca) were used and kept in malt extract agar medium. Agarized synthetic Galvagno GG medium8 was utilized for the screening prior to optimization, replacing asparagine by glutamic acid (9 g/l). The inducers assayed were phenol (0.5 mM), vanillin (0.5 mM), coumarin (0.5 mM), guaiacol (0.5 mM), ferulic acid (0.5 mM), vanillic acid (0.5 mM), coumaric acid (0.5 mM), CuSO4 (0.25 mM), MnCl2 (0.25 mM), CdNO3 (0.25 mM) and Cr2(SO4)3 (0.25 mM). The optimization of laccase production in liquid medium was performed as an incomplete factorial Doehlert design varying the concentrations of peptone and inducers as shown in Table 1. Glucose (40 g/l) was used as a carbon source; yeast extract (10 g/l) was used as a vitamin source, and peptone as a nitrogen source in order to obtain a less expensive optimized medium. All media were sterilized at 121 ºC for 20 min. One ml of each liquid medium was sampled in sterile conditions every 5 days using a 1000 ?l micropipette. Samples were stored in Eppendorf tubes at -20 ºC until measurement.

Quantification of laccase activity
Laccase enzyme activity was measured using 2, 6 dimethoxyphenol (DMP) 5 mM as substrate in Na acetate buffer 0.1 M, pH 3.6. The reaction was carried out in tubes containing 2.5 ml of substrate solution and incubated for 5-10 min in a bath at 30 ºC. The supernantant was added (5-50 ?l) and incubated at different times, and absorbance of the product (cerulignone) was recorded at 469 nm. The reagent without supernatant was used as baseline for absorbance measurements. Enzyme activity corresponding to the screening was measured replacing the aliquots by agar circles of 5 mm diameter of 20-day-old cultures. Enzyme activity is always expressed as UE (?mol of product per min) and, in the case of agarized cultures, they were divided by the weight of the entire agar circles (UE/g).
Electrophoretic separation
Electrophoretic separation was performed in 9% polyacrylamide gels with sodium dodecyl sulfate (SDS) using trypsin (21.5 kDa), carbonic anhydrase (31 kDa), ovalbumin (45 kDa), bovine serum albumin (66.2 kDa) and phosphorylase b (97.4 kDa) as molecular weight markers (Bio-Rad, Hercules, CA, USA). Acrylamide gel was fixed in a mixture of methanol: acetic acid: water (1:1:1) for 5 min, then immersed in a solution of 5 mM DMP in acetate buffer, pH 3.6 and incubated at room temperature (25 °C) until the laccase activity bands appeared. Equivalent laccase activities were inoculated in each well, and therefore, the intensity of the bands did not represent the total activity in the supernatant. Induction was inferred from the activity in the supernatant while electrophoresis only determined the prevalence or the presence of a band in relation to another one. Despite the addition of SDS, laccase did not lose its activity and could be revealed in its substrate DMP.
Thermostability assays
Thermostability was studied as a function of pH in supernatants with 50% citrate buffer 0.5 mM at pH 3, 4, 5 and 6. Aliquots of supernatant of liquid media were incubated (250 ?l) in 1.5 ml. Eppendorf tubes were kept for 12 h in thermal baths and sampling was performed at 10, 20 and 40 min, and then every hour. One Eppendorf tube was extracted each time and activity was quantified as described above. The graphs, along with their corresponding statistical analysis, as well as the calculation of enzyme half-lives were done in GraphPad Prism (GraphPad Software Inc.). Optimization factorial trial was analyzed with STATISTICA (StatSoft Inc.) software.
Results
Effect on laccase inducers
Figure 1 shows induction of both strains on agarized GG medium. Activities correspond to 20-day-old cultures. No appreciable activity was detected in younger cultures. The same pattern is observed in both strains with superiority in E47. In both cases, copper 0.25 mM appeared to be the best inducer, followed by manganese in the same concentration. Phenolic compound (0.5 mM) induction showed to be variable with ferulic acid demonstrating higher induction than other compounds. The results of the separation by SDS-PAGE revealed with DMP are shown in Figure 2. In the controls of both strains only two bands were visible. The lighter band of both strains is between 95 and 99 kDa and the heaviest between 115 and 125 kDa, with slight differences between strains. Copper did not induce expression of new isozymes but increased the production of the lightest isoenzyme of the non-induced cultures (between 95 and 99 kDa). Regarding the induction with aromatic compounds, ferulic and vanillic acids increased the proportion of the lighter band (95-99 kDa). They also induced the production of an additional band, which was lighter than the two of the controls, although the presence of this band is variable in vanillic acid. In both strains this additional lighter band showed a relative weight of 85-90 kDa.
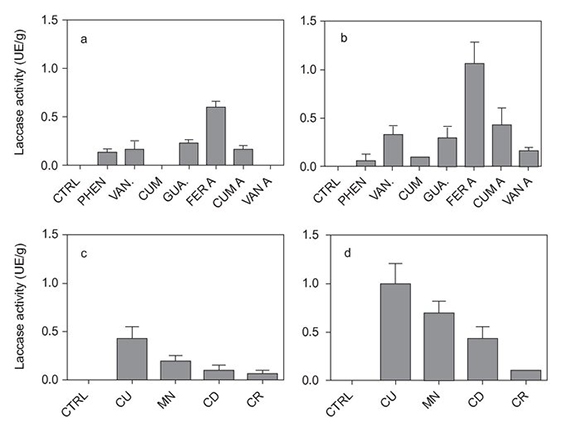
Figure 1. Laccase activity in agar discs of 20-day-old cultures induced with phenolic compounds (0.25 mM) (a: strain S, and b: strain E47) and metallic compounds (0.5 mM) (c: strain S, and d: strain E47). CTRL: control, PHEN: phenol, VAN: vanillin, CUM: coumarin, GUA: guaiacole, FER A: ferulic acid, CUM A: coumaric acid, VAN A: vanillic acid, CU: copper, MN: manganese, CD: cadmium, CR: chromium.

Figure 2. SDS-PAGE of supernatants of induced and non-induced liquid media. mwm: molecular weight marker; fer: ferulic acid; van: vanillic acid; Cu: Copper; Mn: manganese; E: E47 strain; S: strain S.
Optimization of laccase production
Laccase production and reducing sugar concentration were monitored and it was observed that laccase activity begins to increase when the content of reducing sugars is less than 5 g/l (data not shown). The fit to the quadratic equation is shown in Table 2. In both strains a high percentage of variance is explained by the variables. Laccase activity values of supernatants of 40-day-old cultures were divided by the dry weight of the same day to get the values per gram of dry mycelium. Figure 3 shows the optimization results (laccase activity vs. ferulic acid and copper concentration). Peptone factor value was set at the highest tested in order to analyze the inducer interaction, since peptone concentration showed no significant influence on laccase activity. Figure 3a shows the dependence of laccase production of strain S, harvested on day 40, vs. ferulic acid and copper concentrations. Optimal values of both factors were contained within the space of the experiment and they were close to 1mM for both copper and ferulic acid. Figure 3b shows that in strain E47 laccase production strongly depends on ferulic acid and copper concentration. The optimal values of copper concentration were 1-2 mM with low induction in the absence of ferulic acid. Copper effect becomes more marked in the presence of ferulic acid with an optimum close to 1 mM copper in higher concentrations of this aromatic compound. Laccase activity was not detected in media with low concentrations of both compounds simultaneously. Higher ferulic acid concentrations exerted a toxic effect on mycelial growth (data not shown) and thus an optimal concentration of this for strain E47 was not estimated. Unlike strain E47, strain S showed lower dependence on inducer concentration and constitutive production was detectable in non-induced medium.


Figure 3. Production of laccase vs. concentrations of ferulic acid and copper in strains S (a) and E47 (b). Given the limited influence of peptone in the model, only the topology with peptone at a concentration of 40 g/l is shown.
Laccase produced under optimal conditions was subjected to thermal stability studies. Laccase activity half-lives of crude supernatants corresponding to both strains are shown in Figure 4. The results showed a large variation in function of pH. Furthermore, we observed that the enzyme is more stable at pH close to neutrality than in acidic media.

Figure 4. Half-life values (min) of laccase produced by S and E47 strains at different pH values at 70 ºC.
Discussion
In our work, we found three isozyme variants in both strains, which is the number found by Ko et al.13, although in some electrophoretic separations an additional band appears with tenuous and erratic expression. The significance of these variants for possible industrial application is limited, since in optimized cultures laccase production intermediate variant (between 95 and 99 kDa) far exceeds the activity of the light variant (between 85 and 90 kDa) and the heavy variant (between 115 and 125 kDa) of both strains. A multiple band pattern was observed in the majority of white rot fungi, showing in many cases differences in their physical and chemical behavior9,15. It is also interesting to note that the electrophoretic pattern showed differential responses under induction with phenolic compounds and metals, allowing the combination of effects, as demonstrated in this work. The increased production of laccase in response to the addition of aromatic compounds has been shown in several white rot causing fungi21. Laccase induction with veratryl alcohol was reported for another strain of G. lucidum while ferulic acid had no effect4. The same study shows that high concentrations of nitrogen increased up to five times the production of the enzyme. This has not been observed in our work. These results show that the physiological responses of laccase production are very different depending on the fungal strain used.
The choice of a synthetic medium for the screening and the electrophoretical analysis was intended to discard the effect of natural inducers. However, the replacing of nutrient sources by the less expensive alternatives showed to be more efficient in active supernatant production.
MRE (metal responsive elements) have been found in promoter regions of laccase genes. The presence of these elements is correlated with high copper induction of laccase. XRE (xenobiotic responsive elements) inducing the production of laccase in the presence of different aromatic compounds have been found in the same promoter region7,21. The present results suggest that both elements might regulate laccase 90-95kDa, as ferulic acid and copper can separately increase the activity of the laccase band.
Thermostability of laccases produced in the tested culture media is higher than that quoted in the literature. Baldrian5 indicates that G. lucidum laccases are completely inactivated at 60 ºC. Thermal resistance of enzymes of industrial application is evaluated not only at the optimal temperature of activity but at much higher temperatures since it is used as a measure of robustness. In the last years, the need for more robust laccases has forced not only the screening of white rot fungi, but also the modifications of the enzyme in efficient strains to produce a more stable protein with no changes in its activity during the process14. Laccases of our G. lucidum strains have a similar half-life to those with high stability values, such as T. trogii14, Pleurotus sajorcaju22 and Fomes sclerodermeus20. Our results show an inverse behavior to the results in most of the literature on white rot fungi, which has found an increased stability at low pH3. However, there have been exceptions1 and our results fall within those exceptions.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
We thank Gustavo Guajardo for kindly proofreading our English manuscript. This work was supported by CONICET (PIP 413) and UBA (UBACYT 049).
1. Baldrian P. Purification and characterization of laccase from the white-rot fungus Daedalea quercina and decolorization of synthetic dyes by the enzyme. Appl Microbiol Biotechnol. 2004;63:560-3. [ Links ]
2. Baldrian P. Fungal laccases – occurrence and properties. FEMS Microbiol Rev. 2006;30:215-42.
3. Bollag JM, Leonowicz A. Comparative studies of extracellular fungal laccases. Appl Environ Microbiol. 1984;48:849-54. [ Links ]
4. D'Souza TM, Merrit CS, Reddy CA. Lignin-modifying enzymes of the white rot basidiomycete Ganoderma lucidum. Appl Environ Microbiol. 1999;65:5307-13. [ Links ]
5. Elisashvili V, Kachlishvili E, Khardziani T, Agathos SN. Effect of aromatic compounds on the production of laccase and manganese peroxidase by white-rot basidiomycetes. J Ind Microbiol Biotechnol. 2010;37:1091-6. [ Links ]
6. Galhaup C, Haltrich D. Enhanced formation of laccase activity by the white-rot fungus Trametes pubescens in the presence of copper. Appl Microbiol Biotechnol. 2001;56:225-32. [ Links ]
7. Galhaup C, Goller S, Peterbauer CK, Strauss J, Haltrich D. Characterization of the major laccase isoenzyme from Trametes pubescens and regulation of its synthesis by metal ions. Microbiology. 2002;148:2159-69. [ Links ]
8. Galvagno M. Ensayos de nutrición en Ascobolus crenulatus P. Karst (Fungi, Ascomycetes). Bol Soc Argent Bot. 1976;17:95-118. [ Links ]
9. Giardina P, Faraco V, Pezzella C, Piscitelli A, Vanhulle S, Sannia G. Laccases: a never- ending story. Cell Mol Life Sci. 2010;67:369-85. [ Links ]
10. Hess J, Leitner C, Galhaup C, Kulbe KD, Hinterstoisser B, Steinwender M, Haltrich D. Enhanced formation of extracellular laccase activity by the white-rot fungus Trametes multicolor. Appl Biochem Biotechnol. 2002;98-100:229-41. [ Links ]
11. Kiiskinen LL, Kruus K, Bailey M, Ylosmaki E, Siika-Aho M, Saloheimo M. Expression of Melanocarpus albomyces laccase in Trichoderma reesei and characterization of the purified enzyme. Microbiology. 2004;150:3065-74. [ Links ]
12. Kiiskinen LL, Viikari L, Kruus K. Purification and characterization of a novel laccase from the ascomycete Melanocarpus albomyces. Appl Microbiol Biotechnol. 2002;59:198-204. [ Links ]
13. Ko EM, Leem YE, Choi HT. Purification and characterization of laccase isozymes from the white-rot basidiomycete Ganoderma lucidum. Appl Microbiol Biotechnol. 2001;57:98-102. [ Links ]
14. Kucharzyk KH, Janusz G, Karczmarczyk I, Rogalski J. Chemical modifications of laccase from white-rot Basidiomycete Cerrena unicolor. Appl Biochem Biotechnol. 2012;168:1989-2003. [ Links ]
15. Levin L, Forchiassin F, Ramos AM. Copper induction of ligninmodifying enzymes in the white-rot fungus Trametes trogii. Mycologia. 2005;94:377-83. [ Links ]
16. Li P, Wang H, Liu G, Li X, Yao J. The effect of carbon source succession on laccase activity in the co-cultured process of Ganoderma lucidum and a yeast. Enzyme Microb Technol. 2011;48:1-6. [ Links ]
17. Matos AJ, Bezerra RM, Dias AA. Screening of fungal isolates and properties of Ganoderma applanatum intended for olive mill wastewater decolorization and dephenolization. Lett Appl Microbiol. 2007;45:270-5. [ Links ]
18. Mendonca RT, Jara JF, González V, Elissetche JP, Freer J. Evaluation of the white-rot fungi Ganoderma australe and Ceriporiopsis subvermispora in biotechnological applications. J Ind Microbiol Biotechnol. 2008;35:1323-30. [ Links ]
19. Murugesan K, Yang IH, Kim YM, Jeon JR, Chang YS. Enhanced transformation of malachite green by laccase of Ganoderma lucidum in the presence of natural phenolic compounds. Appl Microbiol Biotechnol. 2009;82:341-50. [ Links ]
20. Papinutti L, Dimitri P, Forchiassin F. Stabilization studies of Fomes sclerodermeus laccases. Bioresour Technol. 2008;99:419-24. [ Links ]
21. Piscitelli A, Giardina P, Lettera V, Pezzella C, Sannia G, Faraco V. Induction and transcriptional regulation of laccases in fungi. Curr Genomics. 2011;12:104-12. [ Links ]
22. Zucca P, Rescigno A, Olianas A, Maccioni S, Sollai F, Sanjust E. Induction, purification, and characterization of a laccase isozyme from Pleurotus sajor-caju and the potential in decolorization of textile dyes. J Mol Cat B. 2011;68:216-22. [ Links ]














