Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista argentina de microbiología
versión On-line ISSN 1851-7617
Rev. argent. microbiol. vol.47 no.1 Ciudad Autónoma de Buenos Aires mar. 2015
http://dx.doi.org/10.1016/j.ram.2015.01.005
BRIEF REPORT
http://dx.doi.org/10.1016/j.ram.2015.01.005
Presence of antibodies against Leptospira serovars in Chaetophractus villosus (Mammalia, Dasypodidae), La Pampa province, Argentina
Presencia de anticuerpos contra serovares de Leptospira enChaetophractus villosus (Mammalia, Dasypodidae) en la provincia de La Pampa, Argentina
Marta S. Kina,*, Bibiana Brihuegab, Marcelo Fortc, Fernando Delgadob, Daniel Bedottic, Emma B. Casanaved
a. Chordate Biology, Department of Natural and Exact Sciences, UNLPam, Santa Rosa, La Pampa, Argentina
b. Institute of Pathobiology, CICVyA - CNIA, INTA, Morón, Buenos Aires, Argentina
c. Animal Health Laboratory, INTA, Anguil, Anguil, La Pampa, Argentina
d. Chair Animal Physiology, Department of Biology, Biochemistry and Pharmacy, Universidad Nacional del Sur, CONICET, INBIOSUR, Bahía Blanca, Argentina
* Corresponding author. E-mail address: kinsusana@yahoo.com.ar (M.S. Kin).
Received 2 April 2014; accepted 3 January 2015
Available online 6 March 2015
0325-7541/© 2014 Asociación Argentina de Microbiología. Published by Elsevier España, S.L.U. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Abstract
Leptospirosis is a zoonosis of worldwide distribution. The aim of this study was to examine the presence of antibodies against 21 Leptospira reactive serovars in Chaetophractus villosus in La Pampa province, Argentina, using the microscopic agglutination test (MAT). Pathologic changes compatible with leptospirosis and in situ detection of the agent by immunohistochemistry were studied in 24 and 3 individuals respectively. Only 35/150 (23.3%) serum samples had antibodies against Leptospira sp. Six percent of the samples reacted with serovar Canicola, 4.7% with serovar Castellonis, 1.3% with serovar Icterohemorrhagieae and 0.7% with serovar Hardjo. Sixteen (10.6%) serum samples agglutinated with Castellonis-Icterohemorrhagiae and Canicola-Castellonis serovars, both with 4.7%, and Canicola-Hardjo and Castellonis-Canicola-Icterohemorrhagiae both with 0.6%. Fourteen animals had variable degrees of lesions, which were more severe in animals with higher serological titers (3200), and Leptospira sp. was detected in 3 animals by immunohistochemistry. These results represent the first record of the presence of Leptospira in C. villosus in La Pampa.
Keywords
Leptospira; Serovars; Wildlife; Xenarthra; Chaetopractus villosus.
Resumen
La leptospirosis es una zoonosis de distribución mundial. Nuestro objetivo fue examinar la presencia de anticuerpos contra 21 serovares reactivos de Leptospira en Chaetopractus villosus en la provincia de La Pampa, Argentina, mediante la prueba de aglutinación microscópica (MAT). Se realizó el estudio histopatológico y la detección in situ del agente por inmunohistoquímica en 24 y 3 individuos, respectivamente. Solo 35/150 (23,3%) muestras de suero presentaron anticuerpos contra Leptospira sp. Seis por ciento reaccionaron al serovar Canicola; 4,7% a Castellonis; 1,3% a Icterohemorrhagieae y 0,7% a Hardjo. Dieciséis (10,6%) sueros aglutinaron con Canicola-Castellonis y Castellonis-Icterohemorrhagiae, ambos con 4,7%, y con Canicola-Hardjo y Castellonis-Canicola-Icterohemorrhagiae, ambos con 0,6%. En 14 animales se encontraron lesiones compatibles, las que resultaron más graves en animales con títulos serológicos elevados (3200). En 3 animales estudiados se detectó el agente causal por inmunohistoquímica. Estos resultados constituyen los primeros registros de la presencia de Leptospira en C. villosus en La Pampa.
Palabras clave
Leptospira; Serovares; Chaetopractus villosus; Animales silvestres; Xenarthra.
Leptospirosis is an infectious disease of great public health concern. It is considered the zoonosis with the largest global distribution1. This disease is generally caused by two major pathogenic genomospecies: Leptospira borgpetersenii and Leptospira interrogans sensu stricto3. Each serovar has one or more preferred host animals; however, each animal species can be a host to one or more serovars, whereas humans can be hosts to many serovars.
Domestic and wild animals are important reservoirs of Leptospira, which is always excreted through the urine in a discontinuous way and in varying periods of time. The transmission to humans is the result of exposure to the urine of infected animals by direct contact or through the water. The rural areas are at a greater risk because of the manipulation of domestic and wild animals for meat consumption; armadillos (Xenarthra, Dasypodidae) are a clear example of this situation. Chaetophractus villosus is a member of the superorder Xenarthra, and its distribution range extends from the arid Gran Chaco region, which is located between Bolivia, Paraguay and northern Argentina, to as far south as the Argentine Tierra del Fuego and Magallanes in Chile5. C. villosus is an omnivore that feeds on insects, invertebrates, small vertebrates, seeds and carrion (infected animal tissues such as fetuses and placentas) of infected animals. Leptospiras in C. villosus have been detected only in Buenos Aires province, Argentina. Different serovars have been isolated, including Paidjan, Argentiniensis, Hardjo, Canicola, Bataviae and Leptospira biflexa2,4,8,13,14. In addition, the following serovars were identified by serology: Hardjo, Wolffi, Paidjan, Argentiniensis, Bataviae, Canicola, Sejroe, Hebdomadis, Pomona, Castellonis, Grippotyphosa and Icterohaemorrhagiae4,8.
No updated information has been published since the end of the 70 s, and no publications are available from La Pampa province about Leptospira in C. villosus populations, except for the province of Buenos Aires10,11.
For this reason, the aim of this study is to know the seroprevalence of antibodies against Leptospira serovars in C. villosus from La Pampa province, Argentina, through the microscopic agglutination test (MAT) and to describe the presence of histopathological lesions compatible with the detection of Leptospira by immunohistochemistry.
The capture site is located in central La Pampa, where the weather is characterized by hot, rainy summers with temperatures over 35 °C and cold winters with average temperatures of 10 °C, including frequent and severe ground frosts. Rains are common in spring and autumn, with winter being the driest season. Annual rainfall varies from 450 mm to 800 mm. Beef is the most important production in the region. Management is extensive and cows are free-range year round, with stocking rates depending on the available pasture, which in turn depends on the season and weather conditions. Cattle stocking rates have an average of 0.75 cows per hectare.
C. villosus were captured with permission of the Ministry of Production, Secretariat of Agricultural and Natural Resources Directorate of La Pampa province, and the agreement of farm owners. The armadillos were trapped and carried to the laboratory under adequate care. In order to determine the age, the specimens were measured (from snout to tail tip), and their general appearance was evaluated. Armadillos with a length equal to or less than 460 mm were considered juveniles, whereas longer specimens were classified as adults.
Blood was extracted from the caudal vein of 150 armadillos captured between 2007 and 2010 in La Pampa province. The blood samples were centrifuged for 15 min at 2500 rpm. Sera were separated and stored at -20 °C until the time of analysis and testing for the presence of Leptospira sp. antibodies. The MAT was used in the analysis of antibodies against Leptospira sp., using the following serovars as antigens: Castellonis, Canicola, Celledoni, Icterohaemorrhagiae, Hardjo, Pomona, Grippotyphosa, Pyrogenes, Ranarum, Hebdomadis, Sarmin, Bataviae, Mini, Autumnalis, Cynopteri, Panama, Australis, Javanica, Djasiman, Wolffi and Tarassovi.Initial serum dilution was 1:25, and sera with a titer of 50 or higher were considered positive.
Twenty four C. villosus, with previous serological tests for Leptospira (14 positive and 10 negative) were euthanized under anesthesia (tiletamine and zolazepam, 5.0 mg/kg/I.M), respecting the guidelines of the Canadian Council of Animal Care referring to working with experimental animals used in scientific research. Kidneys were extracted for renal histology in order to look for lesions compatible with leptospirosis and fixed in 10% formaldehyde solution. Then, kidney samples were embedded in paraffin, sectioned and stained with hematoxylin-eosin. Finally, three samples with lesions compatible with leptospirosis infection were used for in situ detection of Leptospira by immunohistochemistry following already described procedures5. Rabbit polyvalent antibody reactive against serovar Canicola, Pomona and Icterohaemorragiae (USDA) was used as conjugate and positive results were revealed with AEC (aminoethylcarbazole solution) as chromogenic substrate. Finally samples were stained with hematoxylin.
The sacrificed animals were deposited in the mammalian collection of the Universidad Nacional de La Pampa (UNLPam MA under the following numbers: UNLPam MA609; UNLPam MA639; UNLPam MA651; UNLPam MA652; UNLPam MA653; UNLPam MA660; UNLPam MA661; UNLPam MA664; UNLPam MA673; UNLPam MA676; UNLPam MA697; UNLPam MA698; UNLPam MA703; UNLPam MA704; UNLPam MA706; UNLPam MA709; UNLPam MA727; UNLPam MA739; UNLPam MA742; UNLPam MA744; UNLPam MA773; UNLPam MA776; UNLPam MA786; UNLPam MA788; UNLPam MA789; UNLPam MA793; UNLPam MA795; UNLPam MA799; UNLPam MA800; UNLPam MA803; UNLPam MA810; UNLPam MA815; UNLPam MA825; UNLPam MA836; UNLPam MA843). It is worth noting that C. villosus is not an endangered species9.
Statistical analyses were performed using the Chi-square test for two variables: age and sex. Statistical significance in this study was defined at the p ≤ 0.05 levels (Epi Info 6.0.4 software).
Out of the 150 C. villosus analyzed, 35 (23.3%) had antibodies against Leptospira (95% confidence intervals 16.5-30.2, Table 1).
Table 1 Agglutination titers according to the MAT (microscopic agglutination test) of different Leptospira serovars reactants (Castellonis, Canicola, Hardjo, Icterohaemorragiae) in C. villosus, segregated by sex (F: female, M: male), age (adults, juvenile) and lesion presence.

a Histopathology was not performed (n/p).
Seventy of the 150 samples corresponded to male specimens, 18 (25.7%) of which were positive for Leptospira, whereas the remaining 80 samples were obtained from females, 17 (21.3%) of which showed positive results. Based on their length, 127 of the C. villosus examined were adults, 34 (26.7) of which had positive results for Leptospira, whereas only 1/23 (4.3%) juveniles showed positive results.
Of the 150 serum samples tested, 6% resulted positive to serovar Canicola, 4.7% to Castellonis, 1.3% to Icterohemorrhagiae and 0.7% to Hardjo. Finally, 10.6% of the serum samples agglutinated with two or more serovars, being the Castellonis-Icterohemorrhagiae and Canicola-Castellonis serovars the most frequently observed patterns, both with 4.7%, and Canicola-Hardjo and Castellonis-Canicola-Icterohemorrhagiae with 0.6%. The highest titers were observed with serovars Canicola, Castellonis (3200) and Icterohemorrhagiae (800). No antibodies were detected against serovars Grippotyphosa, Pomona, Pyrogenes, Ranarum, Celledoni, Hebdomadis, Sarmin, Bataviae, Autumnalis, Cynopteri, Panama, Australis, Mini, Javanica, Djasiman, Wolffi and Tarasovi.
Out of the 24 kidney samples, 10 had no histological lesions (they belonged to individuals with negative serological tests) and 14 kidneys had lesions which belonged to animals that presented specific antibodies to Leptospira, with serological titers from 50 to 3200 for serovars Canicola, Castellonis, Icterohemorrhagiae and Hardjo. Animals which were serologically positive had a gradient of histopathological changes that were more severe in the animals with the highest titers. In the less severe cases the lesions found consisted of infiltrations of mononuclear cells, mostly lymphocytes and plasmatic cells around the renal corpuscles, whereas in the most severe cases the lesions had spread to the rest of the cortex, producing a cortical interstitial nephritis that could compromise the medullary area. In 9 of the 14 animals that tested positive there was a lymphocytic infiltration of plasmatic cells to the level of the submucosa in the renal pelvis. The parietal layer of Bowman's capsule was thicker in the most severe cases, with a retraction of the glomerular tuft and a dilation of the capsular space. In the renal tubules, especially in the collecting duct, there were cylindrical or teardrop-shaped deposits of hyaline material showing different levels of tubular degeneration (Fig. 1). Immunostaining was detected mainly inside the proximal tubules, added to the surface of epithelial cells, and between those cells in the three tested animals (Fig. 2).
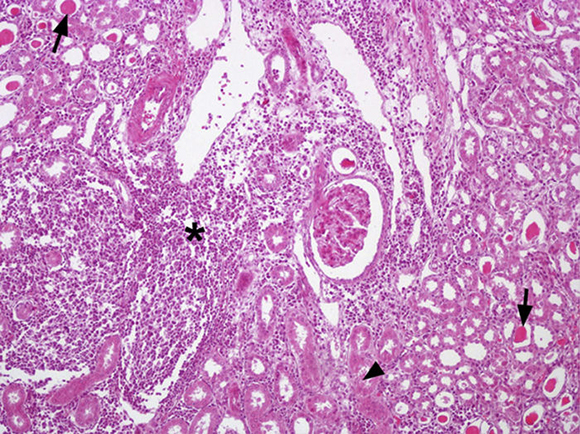
Figure 1 Kidney, multifocal infiltrations of mononuclear cells especially in cortical zone (*) and presence of eosinophilic material in proximal tubules are observed (→) as well as deposits of hyaline material in distal tubules and collecting duct (?). Staining haematoxylin-eosin. 200×.

Figure 2 Kidney, immunostaining can be found as thin fibers between tubular cells and over the epithelial cells (arrows →), and with a diffuse pattern within the tubules (*). The interstitial tissue appeared infiltrated with lymphocytic cells (1). Immunohistochemistry, AEC (solution aminoethylcarbazole) - haematoxylin-eosin. 400×.
The results of this study recognized positive serology of Leptospira in C. villosus in La Pampa province, where serovars Canicola (11.3%) and Castellonis (10%) were more prevalent and Hardjo (1.3%) and Icterohemorragiae (1.3%) were less common. No significant differences of prevalence between males and females (p = 0.519) were found; however, they were significant between juveniles and adults (p = 0.019).
Cuba-Caparó4 identified serovars Hardjo, Wolffi, Sejroe, Hebdomadis, Bataviae and Canicola, with a prevalence of 17.9%. Myers et al8. analyzed 89 C. villosus in Azul (Buenos Aires), resulting in 47.2% of animals that tested positive to different serovars including 1.1% for Pomona, 21.3% for Hardjo, Wolffi, Sejroe, Hebdomadis, 15.7% for Argentiniensis, Paidjan and Bataviae, and 2.2% for Canicola; 6.7% of armadillos reacted to more than one serovar and antibodies against L. biflexa were found in one animal. These authors also mention the presence of serovar Argentiniensis with 11.2%; however, this serovar could not be analyzed in armadillo sera from La Pampa because the strain is not currently available.
Scialfa et al10. analyzed five C. villosus from the province of Buenos Aires that tested negative to serovars Icterohaemoragiae, Canicola, Castellonis, Tarassovi, Pomona, Wolffi, Pyrogenes, Grippotyphosa, Hardjo and Hebdomadis, while another study in 201311 analyzed six C. villosus, two of which tested positive to serovars Canicola, Castellonis, Grippotyphosa and Icterohaemorrhagiae (there was no discrimination of the serovars present in each animal). All samples were negative to serovars Tarassovi, Pomona, Wolffi, Pyrogenes, Hardjo and Hebdomadis.
Motie et al7. mention serovar Canicola for Dasypus novemcinctus in central Florida (EEUU) with a prevalence of 2.4% (7/86). The prevalence found by Myers et al8. (2.2%) was lower than the one found in this study (6%) for C. villosus in La Pampa.
The microscopic lesions observed in the kidneys of C. villosus agree with the ones described for leptospirosis in other armadillos8,12 and farm animals6. Detection of Leptospira in all tested animals confirmed the etiology of detected changes, and suggested that histopathology was useful for diagnosing the disease in the untested armadillos.
C. villosus adults presented the highest prevalence against Leptospira (26.7%) with respect to juveniles (4.3%). The difference could be a consequence of greater exposure time of adults to an environment contaminated with Leptospira.
Our results represent the first record of the presence of antibodies against L. interrogans, Icterohaemorrhagiae, Canicola and Hardjo serovars and antibodies against L. borgpetersenii Castellonis serovar for C. villosus in La Pampa, being the Castellonis and Canicola serovars those with the highest prevalence and the highest titers (3200). The presence of different serovars of pathogenic Leptospira in C. villosus shows a potential risk. Since Leptospira are mainly shed in urine, contaminating water, food and soil, new questions about other domestic and wild species should be answered. Furthermore, the importance of health education should be highlighted in order to raise awareness of the dangers involved in hunting, manipulating and ingesting armadillo meat, especially in areas where these habits are more frequent.
Ethical disclosures
Protection of human subjects. The authors declare that no experiments were performed on humans for this study. Experiments involving animals were performed respecting the guidelines of the Canadian Council of Animal Care, with the authorization of the Ministry of Production of La Pampa province, and with the permission of the farm owners.
Confidentiality of data. The authors declare that no patient data appear in this article.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Conflict of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
The authors are thankful to UNLPam, UNS, INTA for funding this work (Research Grant UNLPam 2009 Res CS 156/09 and Research Project UNLPam 208 Res CS 161/09; PGI 24B152 SGCyT UNS), to the farmers, for allowing access to their rural properties and to all those who collaborated in animal capture. The authors would also like to thank Graciela Romero for technical assistance, Mr Daniel Funes for preparing the tissue slides and PhD Analía Pugener for critically reviewing the text of this manuscript.
1. Brihuega B, Tealdo M, Temas de zoonosis V. Capítulo 19. Importancia de los animales silvestres en la leptospirosis. AAZ; 2011. p. 169-74. [ Links ]
2. Cacchione RA, Cascelli E, Martínez ES, Zuberbuhler J. Leptospirosis en animales silvestres: aislamiento de una cepa de Leptospira canicola de un peludo (Chaetophractus villosus). Rev Med Vet. 1966;47:363-6 [On-line]. [ Links ] http://www.sovergs.com.br/conbravet2008/anais/cd/resumos/R1179-2.pdf
3. Carrizo A, Brihuega B, Etchechoury I, Arese A, Romero S, Gioffré A, Romano MI, Caimi K. Identificación de antígenos inmunorreactivos de Leptospira interrogans. Rev Argent Microbiol. 2009;41:129-33. [ Links ]
4. Cuba-Caparó A. The armadillo in biomedical research. In: Pan American Health Organization PAHO/ACMR 15/17. Fifteenth Meeting of the Advisory Committee on Medical Research. 1976. p. 1-43 [On-line]. [ Links ] http://hist.library.paho.org/English/ACHR/ACMR1517.pdf.
5. Delgado F, Capellino F, Venzano A, Funes D, Blanco Viera FJ, Auteri C, Romero G, Brihuega B. Adaptación de un protocolo de inmunohistoquímica para la detección de Leptospira spp. en muestras de tejido en formaldehído. Rev Cubana Med Trop. 2007;59:14-8. [ Links ]
6. Gardner AL. Orden Cingulata en Wilson y Reeder. Mammal species of the World. A Taxonimic and Geographic Reference. Third Edition Baltimore: The Johns Hopkins University Press; 2005. p. 94-103. [ Links ]
7. Jubb KVF, Kennedy PC. Patología de los Animales Domésticos. Agropecuaria Hemisferio Sur. Tomo II. Cap, vol. 6; 1980. p. 343-404. [ Links ]
8. Motie A, Myers DM, Storrs EE. A serologic survey for leptospires in nine-banded armadillos (Dasypus novemcinctus L.) in Florida. J Wildlife Dis. 1986;22:423-4. [ Links ]
9. Myers DM, Caparo AC, Moreno JP. Aislamiento del serotipo Hardjo y otras leptospiras de armadillos de Argentina. Bol Sanit Panam. 1977;83:56-65 [On-line]. [ Links ] http://hist.library. paho.org/spanish/Bol/v83n1p56.pdf.
10. Ojeda RA, Chillo V, Díaz Isenrath GB, editors. Libro rojo de mamíferos amenazados de la Argentina. Argentina: SAREM; 2012. [ Links ]
11. Scialfa EA, Brihuega B, Morris WE, Recavarren M, Quintana S, Grune S, Romero G, Bolpe J, Schettino M. First isolation of Leptospira interrogans from Conepatus chinga. Afr J Appl Microbiol Res. 2012;1:1-5. [ Links ]
12. Scialfa E, Brihuega B, Venzano A, Morris WE, Bolpe J, Schettino M. First isolation of Leptospira interrogans from Lycalopex griseus (South American gray fox) in Argentina shows new MLVA genotype. J Wildlife Dis. 2013;49:168-72. [ Links ]
13. Stuart BP, Crowell WA, Adams WV, Carlisle JC. Spontaneous renal disease in Louisiana Armadillos (Dasypus novemcintus). J Wildlife Dis. 1977;13:240-4. [ Links ]
14. Szyfres B, Sulzer CR, Galton MM. Nuevo serotipo de Leptospira del grupo Bataviae aislado en la Argentina. Bol Sanit Panam. 1968;64:225-7 [On-line] Links ]library.paho.org/Spanish/BOL/v64n3p225.pdf" target="_blank">http://hist.library.paho.org/Spanish/BOL/v64n3p225.pdf.













