Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista argentina de microbiología
versión impresa ISSN 0325-7541
Rev. argent. microbiol. vol.47 no.3 Ciudad Autónoma de Buenos Aires set. 2015
http://dx.doi.org/10.1016/j.ram.2015.06.003
ORIGINAL ARTICLE
http://dx.doi.org/10.1016/j.ram.2015.06.003
Impact of hydroquinone used as a redox effector model on potential denitrification, microbial activity and redox condition of a cultivable soil
El impacto de la hidroquinona usada como un modelo de efector redox sobre la desnitrificación potencial, la actividad microbiana y las condiciones redox de un suelo cultivable
Elda B.R. Perotti
Laboratorio de Química Biológica I, Facultad de Ciencias Veterinarias, Consejo de Investigaciones, Universidad Nacional de Rosario, Ov. Lagos y Ruta 33, 2170 Casilda, Provincia de Santa Fe, Argentina
E-mail address: eperotti@unr.edu.ar
Received 6 April 2014; accepted 9 June 2015
Available online 9 September 2015
© 2015 Asociación Argentina de Microbiología. Published by Elsevier España, S.L.U. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Abstract
In this microcosm study, we analyzed the effect produced by hydroquinone on the expression of soil biological denitrification, in relation to the redox state of the soil, both in terms of intensity factor (Eh′) and capacity factor (amount of oxidized or reduced compounds).
The supplementation of an Argiudoll soil with hydroquinone decreased the soil apparent reduction potential (Eh′) and soil dehydrogenase activity (formazan production from tetrazolium chloride reduction; redox capacity factor), the relationship between both factors being highly significative, r = 0.99 (p < 0.001). The bacterial population (measured by colony forming units) increased, and the production of N2O was greater (p < 0.001) at 200 and 400 μg/g dry soil doses. Furthermore, there was an inverse relationship between soil dehydrogenase activity and the number of bacteria (r = −0.82; p < 0.05), increased denitrification activity and changes in the CO2/N2O ratio value. These results suggest that hydroquinone at supplemented doses modified the soil redox state and the functional structure of the microbial population. Acetate supplementation on soil with hydroquinone, to ensure the availability of an energy source for microbial development, confirmed the tendency of the results obtained with the supplementation of hydroquinone alone. The differences observed at increased doses of hydroquinone might be explained by differences on the hydroquinone redox species between treatments.
Keywords: Nitrous oxide; Redox conditions; Dehydrogenase activity; Heterotrophic microorganisms; Hydroquinone doses;
Resumen
En este trabajo estudiamos, en condiciones de microcosmos, el efecto que produce la hidroquinona sobre la expresión de la desnitrificación en relación con el estado de óxido-reducción del suelo, en términos de factor de intensidad (Eh′) y de factor de capacidad (cantidad de compuestos oxidados o reducidos).
La suplementación de un suelo argiudol con hidroquinona disminuyó el potencial de reducción aparente (Eh′) y la actividad deshidrogenasa (producción de formazán a partir de la reducción de cloruro de tetrazolio; factor de capacidad redox), la relación entre ambos factores fue altamente significativa, r = 0,99 (p < 0,001). La población bacteriana heterotrófica (medida como unidades formadoras de colonias) aumentó y la producción de N2O fue mayor (p < 0,001) con las dosis de 200 y 400 μg/g de suelo seco. Además se observó una relación inversa entre la producción de formazán y el número de bacterias (r = −0,82; p < 0,05), la actividad desnitrificadora aumentó y se produjeron cambios en el valor del cociente CO2/N2O. Estos resultados sugieren que la hidroquinona, en las dosis empleadas, modificó el estado redox del suelo y la estructura funcional de la población microbiana. La suplementación con acetato en el suelo con hidroquinona, a fin de asegurar la disponibilidad de una fuente de energía para el desarrollo bacteriano, confirmó la tendencia de los resultados obtenidos con la suplementación con hidroquinona solamente. Las diferencias observadas con el incremento en la dosis de hidroquinona podrían explicarse por las diferencias sobre las especies redox de la hidroquinona entre los tratamientos.
Palabras clave: Óxido nitroso; Condiciones redox; Actividad deshidrogenasa; Microorganismos heterótrofos; Dosis de hidroquinona.
Introduction
Soil phenolic compounds and their derivatives are originated in the soil by the degradation of microorganisms, plants and other soil humic substances. In agro-ecosystems they may be added through agrochemicals or their degradation products. Hydroquinone (HQ) is one of the most important phenolic compounds in soil. It is a quinone type found mainly as a lignin constituent25 and together with semiquinones, they are the main redox reactive groups of humic substances in soil.8 Their oxidation, even within the humic substance group, produces semiquinone and quinone, creating a redox cycle, with electron and proton transfer among different electroactive species (QH2 QH Q), minerals and microbial components in soil.16,29 This speciation behavior is conducted by several physicochemical conditions of the system, among them acid-base state and redox state,20 and affect the amount of redox effectors (redox capacity factor) and the apparent redox potential value (Eh′, redox intensity factor). These redox species may act differently upon biological and non-biological processes that involve e-transfers, i.e., denitrification or Fe2+ oxidation.22,28
In certain environments such as water, HQ has a direct toxic effect on microorganisms.4,6 In complex microbial environments, such as in the humic-mineral matrix of soil, HQ interacts with minerals and there is not such a direct effect on microorganisms. HQ is known to be degraded through microbial activity,12,31 to undergo oxidation by light37 or minerals when it is in contact with soil clay-humic structures, and to get involved in humic substance polymerization.13
Denitrification is a process mediated by a great variety of microorganisms which respire nitrate (use NO3− as electron final acceptor), produce NO2− and N oxides among them N2O.11 The relevance of N2O deserves great attention due to the fact that N2O shows high reactivity over the ozone layer. According to estimations made by Revell and his collaborators,26 in the coming years, N2O may be one of the gases having the greatest negative influence on the greenhouse effect. Moreover, the denitrification process is relevant for its negative effect on soil fertility due to the extraction of nitrogen from the soil.11
In soil, microbial respiration generates many redox reactions that transfer e− to NO3-R,35 free radicals and H2O2,10 or to acceptors introduced as triphenyl tetrazolium chloride (TTC), which is reduced to triphenyl formazan (TPF).9 The TPF production was also used to determine the number of heterotrophic microorganisms2 from the microbial dehydrogenase activity since TTC may act as substrate for these enzymes.32,36 In other words, in our study, the same reaction (TTC reduction) may inform on two related process (i) changes in the redox capacity of the system, and (ii) quantify the evolution of the heterotrophic microbial population.
In this research, we studied the pathways through which HQ affects denitrification expression, in relation to soil oxidation–reduction processes in terms of both redox intensity (Eh′) and redox capacity (amount of TPF produced, number of oxidized or reduced compounds). The study was carried out under controlled laboratory conditions, and the parameters assessed were the number of heterotrophic bacteria, dehydrogenase activity, apparent reduction potential (Eh′), and potential N2O and CO2 production in soil. Acetate as carbonated substrate was supplemented.
Materials and methods
Soil treatment
The loamy-clay soil used in this study is a Typic Argiudoll (Peyrano series) from naturalized grassland located in Casilda (province of Santa Fe, Argentina). Soil was sieved through an ASTM N 5 (4000 μm) and taken from the surface layer (0–15 cm) after removing the top surface layer; the natural plant remains were eliminated by hand. The soil was stored at +4 °C for a maximum of 1 week with field-moist soil (25 %). The main properties of the soil included 37 g/kg organic matter, 2.3 g/kg total-N, pH 6.1, and 27 cmolc/kg cation exchange capacity.
Experimental procedure
Soil (10 g oven-dry basis) was introduced into 22 ml-tubes. Humidity was adjusted to 100 % (p/p) using sterilized deionized water and HQ solution stock and acetate solution, depending on the experiment.
Experiment 1 was conducted to study the effects of HQ supplementation alone. The HQ (Sigma-Aldrich® ,CAS number123-31-9) stock solution was added to obtain final concentrations of 0 (control), 100, 200 and 400 microgram per gram of dry soil (μg/g).
Experiment 2 was conducted to study the effects of HQ supplementation in the presence of a source of carbon and electrons, which was done by adding acetate solution (Sigma-Aldrich®, CAS number 127-09-3) 1 milligram per gram of dry soil in each tube with soil. Afterwards, a HQ stock solution was added to obtain final concentrations of 0 (control), 100, 200 and 400 μg/g.
Tubes were capped with Suba Seal stoppers. All of the experimental treatments were prepared in triplicate and the tubes were placed in a dark chamber at 25 °C for 6 days. At this time samples of each treatment were collected and employed to assess the biological tests and physicochemical analysis.
Biological tests
Soil dehydrogenase activity: Dehydrogenase activity was determined by the reduction of TTC according to the procedure proposed by Casida et al.2 Samples of 1 g were placed into a 18 ml-tube; then 0.01 g CaCO3 and 1 ml of 2,3,5-triphenyl tetrazolium chloride (Sigma–Aldrich®, CAS number 298-96-4) in a water solution (3 %) were added. Tubes were incubated at 30 °C in a dark chamber. After 20 h, acetone was added and the soil suspension was agitated and filtered. The red solution was measured at 485 nm and compared with the TPF standard.
Total heterotrophic bacteria count: Samples (1 g) were collected from each treatment and serial dilutions of each subsample were spread on Luria Broth (LB) agar plates, according to the procedure in Fracchia et al.7 The plates were incubated for 48 h at 30 °C in a dark chamber. Total heterotrophic bacteria were counted and expressed as colony forming units (CFUs) per gram of dry soil.
Denitrification activity: Samples (1 g) of each treatment were placed in a 10 ml-tube capped with septa for gas extraction under anaerobic conditions, and 1 ml sterilized water containing KNO3 (1 mg N/g dry soil) was added. The atmosphere of each tube (2′-vacuum) was replaced by N2 to provide anaerobic conditions. N2 (10 %) was replaced by C2H2 to inhibit N2O reductase activity of the soil denitrifying microorganisms.38 Tubes were placed in a dark chamber at 30 °C during 20 h. The headspace atmosphere was sampled to quantify N2O and CO2 production. Gases were analyzed on a gas chromatograph equipped with a thermal conductivity detector (Konik Instruments S.A., Barcelona, Spain) and a stainless steel column packed with Porapak Q and N2 as carrier gas.
Physicochemical analysis
Apparent redox potential (Eh’)20 was measured with a platinum combined electrode (Orion 96-02). The electrode measurements were calibrated using a solution containing 3 mM potassium ferrocyanide, 3 mM potassium ferricyanide, and 100 mM KCl.39 The 242 mV reference electromotive force was added to the actual electromotive force.21
Data treatment
All results are expressed in terms of weight oven-dry soil. The data were statistically analyzed with the variance analysis; when a significant F-value was detected, the Tukey Test was used to separate the treatment means.23 The Pearson correlation coefficient was used to analyze the relationship between biological and physicochemical parameters and HQ doses, and between dehydrogenase activity and CFUs.
Results and discussion
Soil dehydrogenase activity and number of colony forming units
The soil dehydrogenase activity assessed by TTC reduction decreased when the HQ dose was increased (Fig. 1a and b). This was observed in both experiments, i.e., in soil with HQ alone and soil with HQ plus acetate. The highest dose of HQ decreased the TTC reduction (p < 0.05). The soil supplemented with acetate showed, in general, a higher dehydrogenase activity with respect to the soil without acetate. The relationship between soil dehydrogenase activity and HQ doses plus acetate also showed a negative and statistically significant coefficient correlation, r = −0.99 and r = −0.98 (p < 0.001) in soil with HQ alone and in soil with HQ plus acetate, respectively.
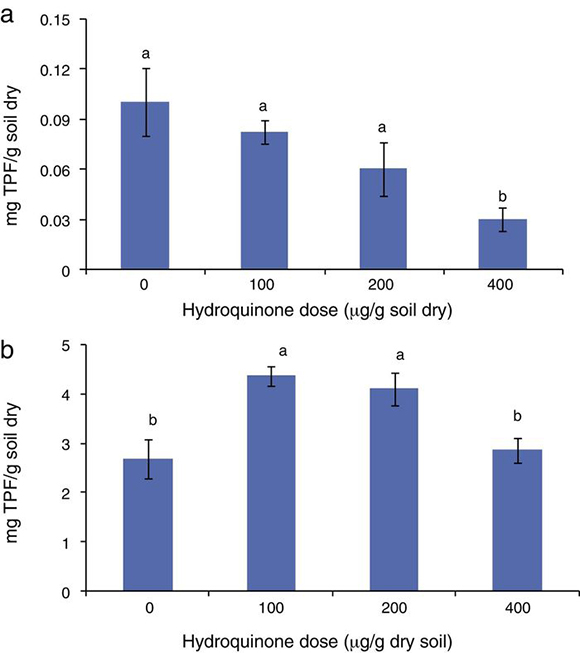
Figure 1. Soil dehydrogenase activity as triphenyl formazan (TPF) production in (a) soil supplemented with hydroquinone alone and (b) soil supplemented with hydroquinone plus acetate (1 mg/g dry soil). Bars labeled with the same letter were not significantly different from each other (p < 0.05, Tuckey's test).
An assessment of HQ supplementation on a soil heterotrophic bacterial population was carried out by counting the CFUs and measuring soil dehydrogenase activity. Although some authors describe HQ as a toxic compound for microorganisms such as Bacillus subtilis in aquatic ecosystems,4 and also for microorganisms in a Chinese fir forest soil,3 the results show that HQ did not reduce CFUs in soil; on the contrary, CFUs increased with HQ supplementation. The units increased from 3.60 × 107 to 1.02 × 108 /g dry soil when 100, 200 and 400 μg HQ per gram of dry soil were added (Fig. 2a).
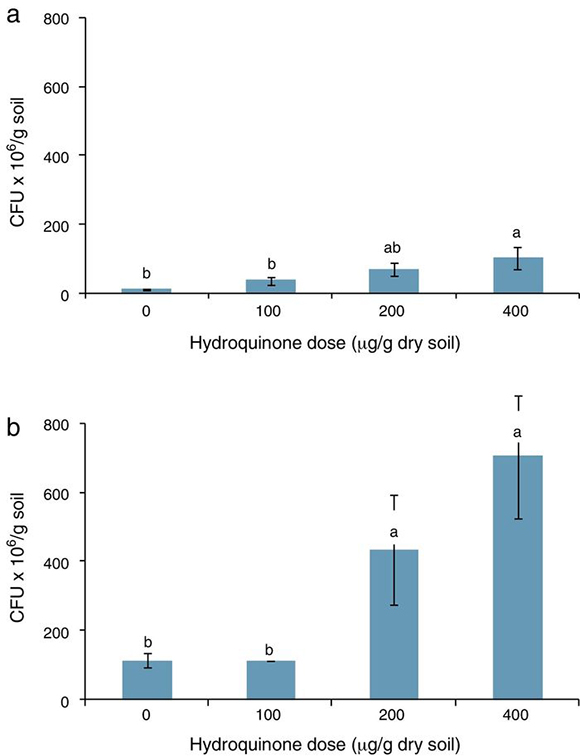
Figure 2. Count of soil heterotrophic bacteria (CFUs) in (a) soil supplemented with hydroquinone alone, and (b) soil supplemented with hydroquinone plus acetate (1 mg/g dry soil). Bars labeled with the same letter were not significantly different from each other (p < 0.05, Tuckey's test).
Moreover, acetate supplementation increased the number of CFUs, which was between 1.1 × 108 and 7.4 × 108 /g dry soil, when 100, 200 and 400 μg per gram of dry soil HQ doses and acetate were added (Fig. 2b). There was a correlation between HQ doses and CFU increase in both experiments, r = 0.81 (p < 0.05) and r = 0.84 (p < 0.05) in experiments 1 and 2, respectively. The number of CFUs was statistically greater (p < 0.05) with higher doses of HQ alone (Fig. 1a) and also with HQ and acetate (Fig. 1b). In complex microbial environments, such as the humic–mineral matrix of soil, HQ may be degraded12 or it may interact with minerals involved in humic substance polymerization,13 and consequently, it does not have a direct negative effect on soil heterotrophic microorganisms.
Soil dehydrogenase activity was described and employed by many authors as an indicator of soil microbial activity.1,9,18,19,34 In this study, the relationship between CFUs and soil dehydrogenase activity was inverse, r = −0.82 (p < 0.05) in soil supplemented with HQ alone and r = −0.79 (p < 0.05) in soil supplemented with HQ plus acetate. These results clearly indicate that in the studied systems, the dehydrogenase activity did not express the activity of the heterotrophic bacterial colony assessed. This result agrees with reports from different authors which indicate, in some cases, that TPF underestimates the actual dehydrogenase activity.17 On the other hand, authors such as Praveen-Kumar and Tarafdar24 showed that not every microorganism colony in soil can reduce TTC. The results obtained in our research are significant because they may show that quinone compounds, which are abundant in soil, are potential effectors in the relationship between TTC reduction and the number of bacteria in soil.
Study of soil redox state
Soil redox intensity, measured by apparent oxidation–reduction potential (Eh′), decreased at the highest HQ dose (400 μg) (Fig. 3). Eh′ values in soil supplemented with HQ alone were microaerophilic conditions,16 between 330 mV at the lowest dose and 265 mV at the highest dose (Fig. 3a). In soil supplemented with acetate, there were greater reduction conditions, between 270 and 0 mV (Fig. 3b). The relationship between these values and HQ concentration provided a negative correlation coefficient, r = −0.99 (p < 0.001) in soil supplemented with HQ alone, and r = −0.82 (p < 0.05) in soil supplemented with HQ plus acetate.
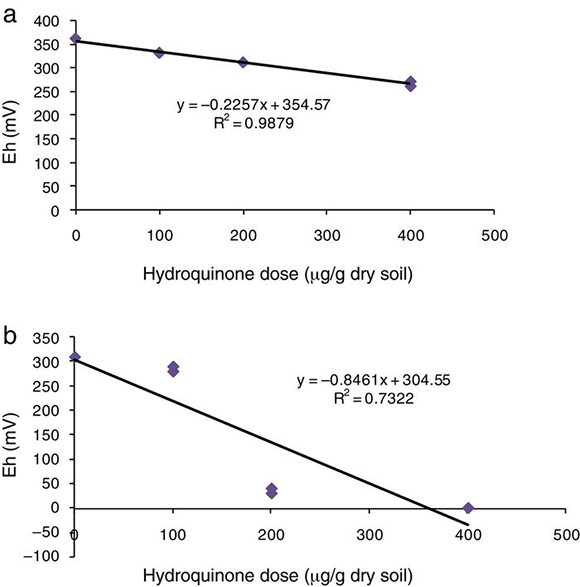
Figure 3. Relationship between apparent redox potential (Eh′) and hydroquinone doses in (a) soil supplemented with hydroquinone alone, and (b) soil supplemented with hydroquinone plus acetate (1 mg/g dry soil).
In addition, the slopes of linear functions (Fig. 3a and b) provided significant data in both experiments, i.e., the figures given by the slope in soil supplemented with HQ alone was −0.22, whereas in soil supplemented with HQ plus acetate it was −0.84. Therefore, the reduction potential was four times lower in soil supplemented with HQ plus acetate than in the treatments of soil supplemented with HQ alone. This shows that the availability of a carbon source such as acetate – which is capable of being easily assimilated – greatly increased soil reduction conditions.
Microbial reduction ability, defined by TPF production as described above (Fig. 1a and b), tended to decrease with the addition of higher HQ doses. Some authors such as Schmidt and Burnett30 described microbial cell reduction ability as the amount of TPF produced in a bacterial culture. In this study, dehydrogenase activity – the amount of formazan produced – becomes a descriptor of soil-redox-capacity-state.
There was a very close relationship between redox intensity (Eh′) and microbial reduction ability (TPF production), r = 0.99 (p < 0.001) and r = 0.72 (p < 0.05) in soil only supplemented with HQ and in those with HQ plus acetate, respectively. This intracellular redox activity requires electron–proton availability to reduce TTC. Therefore, the lower soil dehydrogenase activity observed in the treatments at the highest HQ doses might be due to the fact that (i) the reducing metabolism of the cell might have reduced other compounds27,36 such as quinones or semiquinones, and (ii) in soil supplemented with HQ alone and in soils supplemented with HQ plus acetate a microaerophilic population with respiratory chains that do not use O2 as a terminal electron acceptor might have developed, so that the reduction-power produced was lower than the reducing power of TTC, and consequently, TTC reduction was also low.
Effect on potential denitrification
Soil N2O production caused by NO3− reduction results mainly from microbial denitrification activity. N2O production was measured by introducing an amount of KNO3 which would not limit the activity expression, so as to quantify the potential expression of denitrifying microorganisms under potential conditions14 (see Materials and methods section). NO3− acts as a terminal electron acceptor in denitrifying microorganisms in the absence of O2 and in the presence of acetylene the final product is N2O.33
As may be observed in Fig. 4a, N2O production increased when the HQ doses were higher (p < 0.05) and the acetate supplementation increased the N2O production as observed by Lescure et al.15 When comparing N2O production values in treatments without and with acetate supplementation (Fig. 4a and b, respectively), the addition of this carbonated source increased N2O production approximately 80–95 % in accordance with the HQ dose added.
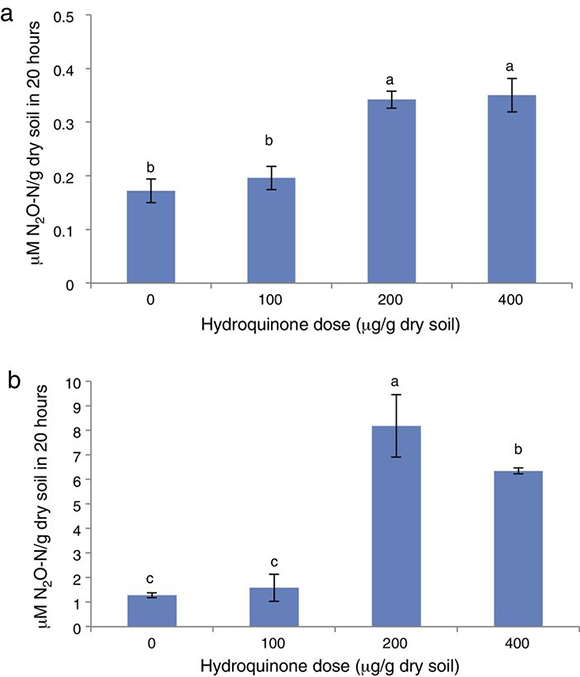
Figure 4. Soil N2O production in (a) soil supplemented with hydroquinone alone and (b) soil supplemented with hydroquinone plus acetate (1 mg/g dry soil). Bars labeled with the same letter were not significantly different from each other (p < 0.05, Tuckey's test).
In addition, in systems supplemented with HQ plus acetate, the treatment with 200 μg/g showed the greatest N2O production, which was up to four times greater than the control without HQ (Fig. 4a), and the treatment at the highest HQ dose (400 μg/g) showed a lower N2O production, although greater than the control and the treatment with 100 μg/g (Fig. 4b).
Carbon dioxide production in soil supplemented with 200 μg/g HQ plus acetate was slightly greater than in the other treatments (p > 0.05) (Table 1). When CO2 production was compared in the experimental soils, with and without acetate, this compound seems to have increased CO2 production, which was four times greater than in the non-supplemented experimental soil. This increase in C-reduction activity may be caused by the higher number of heterotrophic bacteria (CFUs) observed (Fig. 2a and b).
Table 1. CO2 production and CO2/N2O ratio in soil supplemented with hydroquinone 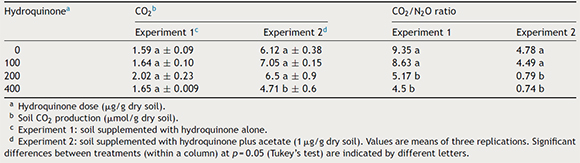
Table 1 shows CO2/N2O ratios obtained from samples of Experiments 1 and 2. The ratio represents the stoichiometric relationship between N2O and CO2 production in a denitrification process, with a theoretical value that corresponds to each electron-donor compound. In this study, the main supplemented electron source was acetate and the theoretical value of this stoichiometric ratio is two.5 If the values differ from this theoretical value, the ratio shows that there are differences in intensity and/or diversity in metabolic pathways that may produce these gases, including the biological denitrification process.21 Table 1 shows that in both experiments, the ratio was lower when the HQ dose was higher, and that an acetate supplementation lowered the ratio value in relation to the treatments without acetate. This suggests that a greater NO3− reduction (Fig. 4a and b) will not depend directly on a higher electron availability provided by an exogenous carbon source. On the other hand, as CO2 values were relatively constant in the treatments with and without acetate, the systems may be showing that electron sources behave differently for NO3− reduction. Those compounds might have come from fermentation routes stimulated by the presence of HQ or from self-oxidation of supplemented HQ, whose oxidized end product might not be CO2. This may explain why CO2 production was relatively constant and N2O production was greater when the HQ concentration was increased.
The supplementation of HQ in the studied soil affected the reduction conditions of the system in relation to redox intensity (Eh′) and reduction capacity (TPF production), and with the HQ supplementation plus acetate in soil these results were confirmed. An inverse relationship between TPF production and the number of microorganisms (CFUs), as well as changes in the CO2/N2O ratio value may confirm that the HQ supplementation modified the functional structure of the microbial population. In addition, HQ supplementation favored the development of a heterotrophic population (shown by CFUs) and the expression of a microbial population, such as the denitrifying population, which does not use O2 as terminal electron acceptor.
Finally, as the supplementation of acetate was carried out in a single concentration – the addition of carbon and energy for the functional biomass was similar in every HQ dose – the changes observed when the supplemented HQ dose was increased may be explained by changes in HQ speciation, which might modify HQ ability to intervene in electron transfer processes such as denitrification, CO2 production and dehydrogenase activity in soil.
Ethical disclosures
Protection of human and animal subjects
The author declares that no experiments were performed on humans or animals for this study.
Confidentiality of data
The author declares that no patient data appear in this article.
Right to privacy and informed consent
The author declares that no patient data appear in this article.
Conflict of interest
The author declares to have no conflicts of interest.
Acknowledgements
I am grateful to Prof. Dr. Alejandro Pidello for stimulating discussion and manuscript revision, and to Mr. Facundo Ferrari for technical assistance. I am also grateful to anonymous reviewers because their comments improved the manuscript, and to Mrs. Mariela Bernacchia for reviewing the English style. This work was supported by the Consejo de Investigaciones de la Universidad Nacional de Rosario (CIUNR) and grants from Secretaría de Ciencia y Técnica de la Universidad Nacional de Rosario (SECYT-UNR).
1. Alef K. Dehydrogenase activity. In: Alef K, Nannipieri P, editors. Methods in applied soil microbiology and biochemistry. Academic Press: London; 1995. p. 228-33. [ Links ]
2. Casida LE, Klein DA, Santoro T. Soil dehydrogenase activity. Soil Sci. 1964;98:371-6. [ Links ]
3. Chen HL, Yao J, Wang F, Choi MMF, Bramanti E, Zaray G. Study on the toxic effects of diphenol compounds on soil microbial activity by a combination of methods. J Hazard Mater. 2009;167:846-51. [ Links ]
4. Chen H, Yao J, Wang F, Chou Y, Chen K, Zhuang R, ChoiMMF, Zaray G. Toxicity of three phenolic compounds and theirmixtures on the gram-positive bacteria Bacillus subtilis in the aquatic environment. Sci Total Environ. 2010;408:1043-9. [ Links ]
5. Delwiche CC. The nitrogen cycle and nitrous oxide. In: Delwiche CC, editor. Denitrification, nitrification, and atmospheric nitrous oxide. New York: Wiley-Interscience; 1981. p. 1-15. [ Links ]
6. Enguita FJ, Leitão AL. Hydroquinone: environmental pollution, toxicity, and microbial answers. BioMed Res Int. 2013:542168, http://dx.doi.org/10.1155/2013/542168 [Epub 2013 Jul 15].
7. Fracchia L, Perotti EB, Pidello A, Rinaldi M, Martinotti MG. Persitence and impact of the PGPR Pseudomonas fluorescens 92 on native microbial communities of biosolids and soil amended with them. J Environ Sci Eng. 2001;5:578-95. [ Links ]
8. Geoffrey D, Ghabbour E. Humic acids: marvelous products of soil chemistry. J Chem Educ. 2001;78:1609-14. [ Links ]
9. Gil-Sotrés F, Trasar-Cepeda C, Leirós MC, Seone S. Different approaches to evaluating soil quality using biochemical properties. Soil Biol Biochem. 2005;37:877-87. [ Links ]
10. Gómez-Toribio V, García-Martín AB, Martínez MJ, Martínez AT, Guillén F. Induction of extracellular hydroxyl radical production by white-rot fungi through quinone redox cycling. Appl Environ Microbiol. 2009:3944-53. [ Links ]
11. Graham Ch J, van Es HM, Melkonian JJ. Nitrous oxide emissions are greater in silt loam soils with a legacy of manure application than without. Biol Fertil Soils. 2013;49:1123-9. [ Links ]
12. Harbison KG, Belly RT. The biodegradation of hydroquinone. Environ Toxicol Chem. 1982;1:9-15. [ Links ]
13. Kung KH, McBride MB. Electron transfer processes between hydroquinone and Hausmannite (Mn3O4). Clays Clay Miner. 1988;34:297-302. [ Links ]
14. Lensi R, Lescure C, Steinberg C, Savoie JM, Faure G. Dynamics of residual enzyme activities, denitrification potential, and phys-icochemical properties in asterilized soil. Soil Biol Biochem. 1988;23:367-73. [ Links ]
15. Lescure C, Menéndez L, Lensi R, Chalamet A, Pidello A. Effect of addition of various carbon substrates on denitrification in a vertic Mollisol. Biol Fertil Soils. 1992;13:125-9. [ Links ]
16. McBride MB. Environmental chemistry of soils. New York: Oxford University Press; 1994. [ Links ]
17. Nannipieri P, Grego S, Ceccanti B. Ecological significance of the biological activity in soil. In: Bollag JM, Stotzky G, editors. Soil biochemistry, vol. 6. Marcel Dekker: New York; 1990. p. 293-355. [ Links ]
18. Novak B, Kubat J. On the relation between dehydrogenase activity and CO2 evolution of soil. Zentrabl Bakteriol Parasitenkd. 1972;12:246-52. [ Links ]
19. Perotti Ebr, Pidello A, Leyendeker L, Trasar-Cepeda C, Leirós MC, Gil-Sotres F. Behaviour of enzymatic activities and root elongation in Argiudolls from the Argentine Humid Pampa treated with biosolids. Rev Argent Microbiol. 2008;40: 120-3. [ Links ]
20. Pidello A. Principes de chimie redox en écologie microbienne. Editions Quae. Coll. Syntheses; 2014. p. 144.
21. Pidello A, Menéndez L, Perotti Ebr. Saccharidic compounds as soil redox effectors related with potential N2O production. Biol Fertil Soils. 1996;23:173-6. [ Links ]
22. Piepenbrock A, Schöder CR, Kappler A. Electron transfer from humic substances to biogenic and a biogenic Fe(III) oxyhydroxide minerals. Environ Sci Technol. 2014;48:1656-64. [ Links ]
23. Pimentel Gomez F. Curso de estadística experimental. Buenos Aires, Argentina: Editorial Hemisferio Sur; 1978. [ Links ]
24. Praveen-Kumar Tarafdar JC. 2,3,5-Triphenyltetrazolium chloride (TTC) as electron acceptor of culturable soil bacteria, fungi and actinomycetes. Biol Fertil Soils. 2003;38:186-9. [ Links ]
25. Reale S, di Tullio A, Spreti N, de Angelis F. Mass Spectrom Rev. 2004;23:87-126. [ Links ]
26. Revell LE, Bodeker GE, Huck PE, Wiliamsom BE, Rozanov E. The sensitivity of stratospheric ozone changes through the 21st century to N2O and CH4. Atmos Chem Phys. 2012;12:11309-17. [ Links ]
27. Rich PR, Mischis LA, Purton S, Wiskich JT. The sites of interaction of triphenyltetrazolium chloride with mitochondrial respiratory chains. FEMS Microbiol Lett. 2001;202:181-7. [ Links ]
28. Richardson DJ. Bacterial respiration: a flexible process for a changing environment. Microbiology. 2000;146:551-71. [ Links ]
29. Scott DT, McKnight DM, Blunt-Hurris EL, Kolesar SA, Lovley DR. Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms. Environ Sci Technol. 1998;32:2984-9. [ Links ]
30. Schmidt EG, Burnett GW. Measurement of the reducing activity of heteroand homofermentative lactobacilli. J Dent Res. 1956;35:370-6. [ Links ]
31. Szewzyk U, Schink B. Degradation of hydroquinone, gentisate, and benzoate by a fermenting bacterium in pure or defined mixed culture. Arch Microbiol. 1989;151:541-5. [ Links ]
32. Tabatabai MA. Soil enzymes. In: Page AL, Miller RH, Keeney DR, editors. Methods of soil analysis: II. Chemical and microbiological properties, Agronomy 10. 2nd ed. Madison, WI: American Society of Agronomy; 1982. p. 903-47. [ Links ]
33. Tiedje JM, Sexstone AJ, Parkin TB, Revsbech NP. Anaerobic processes in soil. Plant Soil. 1984;76:197-212. [ Links ]
34. van Mersi W, Schinner F. An improved and accurate method for determining the dehydrogenase activity of soils with iodonitrotetrazolium chloride. Biol Fertil Soils. 1990;11:216-20. [ Links ]
35. van Trumpe JI, Coates JD. Oxidation of humic substances supports denitrification reactions in agricultural soils. In: American Geophysical Union, Fall Meeting 2007. 2007. Abstract B23D1582. [ Links ]
36. Wolinska A, Stepniewska Z. Dehydrogenase activity in the soil environment. In: Canuto RA, editor. Dehydrogenases. InTech; 2012., http://dx.doi.org/10.5772/48294. ISBN: 987953-307-019-3, Available from: http://www.intechopen.com/books/dehydrogenases/dehydrogenase-activity-in-the-soil-environment.
37. Zhao X, Zhou L, Li R, Xie C, Li S. Evaluation on environmental effect of the urease inhibitor hydroquinone. Chin J Appl Ecol. 1991;2:316-22. [ Links ]
38. Yoshinari T, Hynes R, Knowles R. Acetylene inhibition of nitrous oxide and measurement of denitrification and nitrogen fixation in soil. Soil Biol Biochem. 1977;9:177-83. [ Links ]
39. ZoBell CC. Studies on the redox potential of marine sediments.Bull Am Assoc Pt Geol. 1946;30:477-513. [ Links ]














