Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista argentina de microbiología
versión impresa ISSN 0325-7541versión On-line ISSN 1851-7617
Rev. argent. microbiol. vol.48 no.3 Ciudad Autónoma de Buenos Aires set. 2016
http://dx.doi.org/10.1016/j.ram.2016.04.005
ORIGINAL ARTICLE
http://dx.doi.org/10.1016/j.ram.2016.04.005
Evaluation of the antimicrobial efficacy of Minthostachys verticillata essential oil and limonene against Streptococcus uberis strains isolated from bovine mastitis
Evaluación de la eficacia antimicrobiana del aceite esencial de Minthostachys verticillata y limoneno contra cepas de Streptococcus uberis aislados de mastitis bovina
Ivana D. Montironi, Laura N. Cariddi, Elina B. Reinoso*
Departamento de Microbiología e Inmunología, Universidad Nacional de Río Cuarto, Ruta 36 Km 601, CP 5800 Río Cuarto, Córdoba, Argentina
Received 7 October 2015; accepted 18 April 2016
Available online 9 September 2016
* Corresponding author. E-mail address: ereinoso@exa.unrc.edu.ar (E.B. Reinoso).
0325-7541/© 2016 Asociación Argentina de Microbiología. Published by Elsevier España, S.L.U. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Abstract
Bovine mastitis is a disease that causes great economic losses per year, being Streptococcus uberis the main environmental pathogen involved. The aim of the present study was to determine the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) of Minthostachys verticillata essential oil and limonene for S. uberis strains isolated from bovine mastitis. In addition, the effect of MIC on biofilm formation was analyzed. MIC values for the essential oil ranged from 14.3 to 114.5 mg/ml (1.56–12.5% v/v) and MBC between 114.5 and 229 mg/ml (12.5–25% v/v). MICs for limonene ranged from 3.3 to 52.5 mg/ml (0.39–6.25% v/v) and MBC was 210 mg/ml (25% v/v). Both compounds showed antibacterial activity and affected the biofilm formation of most of the strains tested. In conclusion, these compounds could be used as an alternative and/or complementary therapy for bovine mastitis caused by S. uberis.
Keywords
Streptococcus uberis; Minthostachys verticillata; Essential oil; Limonene; Bovine mastitis; Antibacterial activity.
Resumen
La mastitis bovina es una enfermedad que causa grandes pérdidas económicas por año, Streptococcus uberis es el principal patógeno ambiental involucrado. El objetivo del presente estudio fue determinar la concentración inhibitoria mínima (CIM) y la concentración bactericida mínima (CBM) del aceite esencial de Minthostachys verticillata y del limoneno sobre cepas de S. uberis aisladas de mastitis bovina. Además, se analizó el efecto del aceite esencial y el limoneno en la CIM determinada en caso sobre la formación de biofilm de estas cepas. Los valores de CIM del aceite esencial oscilaron entre 14,3 y 114,5 mg/ml (1,56%-12,5% v/v) y los de CBM entre 114,5 y 229 mg/ml (12,5%-25% v/v). Las CIM del limoneno oscilaron entre 3,3 y 52,5 mg/ml (0,39% - 6,25% v/v) y la CBM fue de 210 mg/ml (25% v/v). Ambos compuestos mostraron actividad antibacteriana y afectaron la formación de biofilm de la mayoría de las cepas. En conclusión, estos compuestos podrían ser utilizados como terapia alternativa o complementaria para la mastitis bovina causada por S. uberis.
Palabras clave
Streptococcus uberis; Minthostachys verticillata; Aceite esencial; Limoneno; Mastitis bovina; Actividad antibacteriana.
Introduction
Mastitis is a worldwide disease of dairy cattle that is caused by a wide variety of organisms that affect milk quality and yield, resulting in major economic losses. In many countries it is the most costly disease in dairy milk production18. Streptococcus uberis is an important pathogen implicated in bovine mastitis, which is predominantly associated with subclinical and clinical intramammary infections in both lactating and non-lactating cows. This species is particularly problematic due to the fact that it is ubiquitous in the dairy environment. A potential virulence factor, possibly linked to the ability of S. uberis to adhere to cells, would be the formation of biofilm33. It is important to forestall the formation of biofilm in order to treat and prevent intramammary infections.
The ineffectiveness of the different procedures to reduce the rate of new infections has directed research studies toward the search for alternative control methods21,27. Within this context, the search for new effective natural prototypes for the treatment of bovine mastitis does not compromise the milk quality that is important for a better quality of dairy farming and food production. Alternative treatments with medicinal plants may be a safe, efficient and a low-cost option for treating bovine mastitis23. Essential oils classified as GRAS (Generally Regarded As Safe), show antibacterial properties and resistance has not been reported after prolonged exposure. Therefore, the investigation of their antimicrobial activity against bacterial agents of mastitis is justifiable9.
Minthostachys verticillata (Griseb) Epling (Lamiaceae), commonly referred to as "peperina", is an ethnobotanical aromatic herb with various uses and properties. This species is distributed in South American countries such as Colombia, Venezuela, Brazil, Ecuador, Peru, Bolivia, and in the northwest and central regions of Argentina24,29. According to folk traditional medicine it is used as a digestive, sedative, antispasmodic, stimulant, and also to alleviate respiratory illnesses, bronchitis, and asthma10.
Moreover, numerous in vitro studies have described the antiviral, antibacterial and antifungal properties of M. verticillata essential oil (EO)2,11,14,20,26.
In addition, the lack of toxic effect of M. verticillata EO and its main compounds, both in vitro as in vivo, has been demonstrated5,7,12,13,32. In a previous assay, we demonstrated that EO obtained from this species and from limonene, one of its main compounds, showed antimicrobial activity against the major bovine mastitis pathogens such as Staphylococcus aureus, S. uberis, Escherichia coli and coagulase negative Staphylococcus (CNS) by the disk diffusion method6.
The aim of the present work was to determine the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) of M. verticillata EO and limonene for S. uberis strains isolated from bovine mastitis. In addition, the effect of MIC on biofilm formation was analyzed.
Materials and methods
Plant material
Green leaves and thin stems from M. verticillata were collected in Villa Larca city, province of San Luis, Argentina in April, 2013. The voucher specimens were deposited in the herbarium of Universidad Nacional de Río Cuarto (Río Cuarto city, province of Córdoba, Argentina).
Essential oil extraction
Essential oil was obtained from the aerial parts of the plant, composed of leaves and parts of the stem. To prepare the EO, 60 grams of ground material were hydrodistilled using a Clevenger-type apparatus for 3 h. The oil was separated from the aqueous phase, dried over anhydrous Na2SO4 and stored in the dark at −20 °C until use30.
The pure compound limonene was purchased from Sigma Aldrich (St. Louis, USA) as (R)-(+)-Limonene.
Identification and quantification of essential oil compounds by gas chromatography–mass spectrometry (GC/MS)
To perform this analysis, a gas chromatograph Clarus 600, PerkinElmer (Shelton, Connecticut, USA) serial number 664N9100105 fitted with a DB5 capillary column (60 m, 0.25 mm ID, 0.25 μm particle), was used. Carrier gas: helium (49.6 psi). Oven temperature program: initial temperature 60 °C (2 min), ramp: 5 °C/min, final temperature 240 °C (10 min). Injector temperature was 300 °C. The sample was injected in split mode (20 ml/min). The chromatogram was obtained in "scan" mode, since m/z = 30 to m/z = 450 (scan time: 0.2 s, inter-scan time: 0.1 s, solvent delay: 5 min). The identification of the compounds present in the EO sample was performed by comparison of retention times and mass spectrum of the components found with the mass spectrum of the program library NIST MS Search 2.0. Under the same chromatographic conditions, the pure compounds (standard grade) were injected to verify the identity of the major components in the sample. Quantification of components present in the oil sample was carried out by measuring the area under each peak of the chromatogram4,34.
Microorganisms
Fifteen S. uberis strains isolated from cows with mastitis from the central dairy region of Argentina were used in this study. The strains were previously identified according to Jayarao et al.15 and additionally confirmed by RFLP analysis of the 16S rRNA gene according to Khan et al.16 All strains were maintained at −20 °C as stock strains in tryptic soy broth (TSB) (Britania S.A., Argentina) with glycerol until use.
MIC and MBC assay
The microdilution method was employed to determine the MIC and the MCB as recommended by the National Committee for Clinical Laboratory Standards8. For inoculum preparation, each strain was grown in 3 ml of TSB for 24 h at 37 °C. The microorganisms to be tested were prepared by dilutions from cultures grown on TSB (1 × 108 colony forming units (CFU/ml), resulting in a bacterial suspension equivalent to 1 × 106 CFU/ml. EO and limonene were twofold serially diluted volume to volume in dimethylsulfoxide (DMSO) (Sintorgan, Buenos Aires, Argentina) and phosphate buffer saline pH 7.4 (PBS) (1:8) to facilitate solubility in the culture medium, and serial dilutions were performed, resulting in concentrations from 229 (25% v/v) to 0.45 mg/ml (0.049% v/v) and 210 (25% v/v) to 0.41 mg/ml (0.049% v/v) for EO and limonene, respectively.
The antimicrobial activity of limonene naturally present in the EO was also evaluated. A volume of 75 μl of each diluted agent and an equal volume of the standardized culture of each strain (1 × 106 CFU/ml) to be tested were added aseptically into the microplates, which were incubated at 37 °C for 24 h. In addition, wells containing each microorganism culture in TSB without the tested agents were measured as positive control, DMSO as vehicle control and only TSB as negative control. The MIC was determined as the lowest concentration of EO or limonene inhibiting visible growth. Absorbance was read at 560 nm using a spectrophotometer (Labsystem Multiskan MS, Thermo, Vantaa, Finland). The percentage of inhibition for the MIC of each agent was calculated using the formula described by Aiemsaard et al.1:
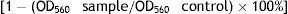
All experiments were repeated twice using different microplates on each occasion.
To determine the MBC, volumes of 100 μL from wells without visible bacterial growth after 24 h of incubation were inoculated onto the surface of tryptic soy agar (TSA) (Britania S.A.) and incubated at 37 °C for 24 h. The MBC was determined as the lowest agent concentration that killed greater than 99.9% of the initial bacterial population, which was indicated by no visible bacterial growth on the TSA plate surfaces. The positive growth of each microorganism culture in TSB without the tested agents served as a positive control and the growth was demonstrated only by TSB. In addition, oil diluent was used as vehicle control.
Antibacterial activity of essential oil and limonene against produced S. uberis biofilms
Seven strains, previously assayed for biofilm formation28,31 and considered to be strong formers according to Stepanovic et al.31 were used in this study. After aerobically incubation at 37 °C for 24 h in order to produce biofilm, the medium was gently removed and the wells were washed three times with PBS pH 7.4. The MICs of the EO and limonene were then added separately to the biofilms and incubated for a further 24 h at 37 °C. After biofilm formation, the medium was aspirated and non-adherent cells were removed by washing the biofilms twice with PBS. The adherent bacteria were stained with 100 μl of 0.1% of crystal violet for 15 min at room temperature. After rinsing with 200 μl of distilled water, the dye bound to the cells was extracted with 200 μl of 99% ethanol for 20 min. The extracted dye was then quantified by measuring absorbance at 560 nm. A series of biofilm oil-free wells and medium alone were also included to serve as positive and negative controls, respectively.
The percentage of biofilm inhibition for each agent was calculated using the formula described by Aiemsaard et al.1
Statistical analysis
All the tests were performed in duplicate. The data obtained by microdilution assays were evaluated to a one-way analysis of variance (ANOVA) and the Tukey's multiple comparison tests using the program GraphPad Prism version 5.00.288 (San Diego, USA, 2007). MIC values (OD) were expressed as mean ± standard deviation (SD). P values <0.05 were considered significant.
Results and discussion
The aim of this study was to determine the antimicrobial efficacy of M. verticillata EO and limonene on S. uberis strains isolated from bovine mastitis.
The yield of M. verticillata EO was 4.8% w/v. GC/MS analysis revealed that the main components of the oil were pulegone (51.7%) and menthone (37.8%). Other compounds present were cis-menthone (1.4%), piperitone (1.4%) and limonene (1.2%). (Fig. 1, Table 1). This work has demonstrated that M. verticillata EO is mainly composed of pulegone and menthone, in agreement with previous studies3–5,11,12,34 where the composition of M. verticillata EO was reported.
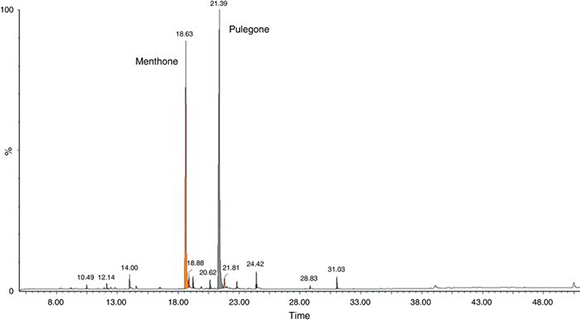
Figure 1. Chromatographic profile of the essential oil from the species Minthostachys verticillata collected from Villa Larca, San Luis, Argentina, obtained by GC–MS. The area represented by the peaks corresponds to the proportions in which each component is in the mixture. Retention times of each peak are observed.
Table 1. Chemical composition of essential oil obtained from Minthostachys verticillata 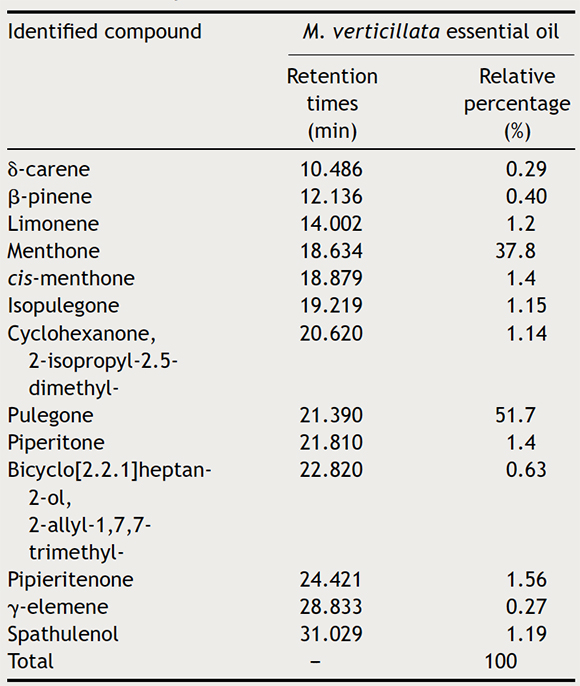
The antimicrobial activity of M. verticillata EO and limonene against S. uberis strains isolated from bovine mastitis has not been previously reported. This is the first study that demonstrated significant antimicrobial activity of both EO and limonene against this bacterium. MIC values for EO ranged from 14.3 to 114.5 mg/ml (1.56–12.5% v/v) and MBC values, between 114.5 and 229 mg/ml (12.5–25% v/v). MICs for limonene ranged from 3.3 to 52.5 mg/ml (0.39–6.25% v/v) and MBC was 210 mg/ml (25% v/v). The MIC values were corroborated by absorbance measurement. Table 2 shows MIC and MBC values (% v/v and OD) of EO and limonene against the seven S. uberis strains that were more sensitive to these compounds. Our results demonstrated that EO as limonene showed good antibacterial activity. The bacterial strains tested have shown susceptibility to both agents.
Table 2. Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values of Minthostachys verticillata essential oil and limonene aganist Streptococcus uberis strains 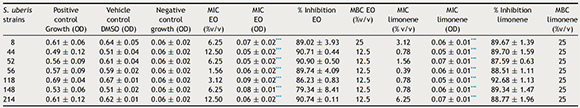
Positive control growth, each strain growth with Trypticase Soy Broth; Negative control growth, Trypticase Soy Broth alone; DMSO, dimethylsulfoxide; MIC, minimum inhibitory concentration; EO, Minthostachys verticillata essential oil; OD, optical density; MBC, minimum bactericidal concentration.
*** - p < 0.0001 respect to positive control (ANOVA and Tukey's test).
Limonene showed inhibitory effects against the strains tested; these results indicated that limonene could be one of the compounds responsible for the antibacterial effect showed by EO. However the whole essential oil demonstrated to be a more potent bacterial agent compared to limonene19.
The EO and limonene were also tested against S. uberis strains considered to be strong biofilm formers. Both agents were able to reduce biofilm production. The percentages of inhibition of EO MIC were from 88.25 ± 7.62 to 23.50 ± 16.26 and of limonene were from 92.18 ± 4.78 to 23.20 ± 16.05. Fig. 2 shows the effectiveness of EO and limonene against S. uberis biofilm.
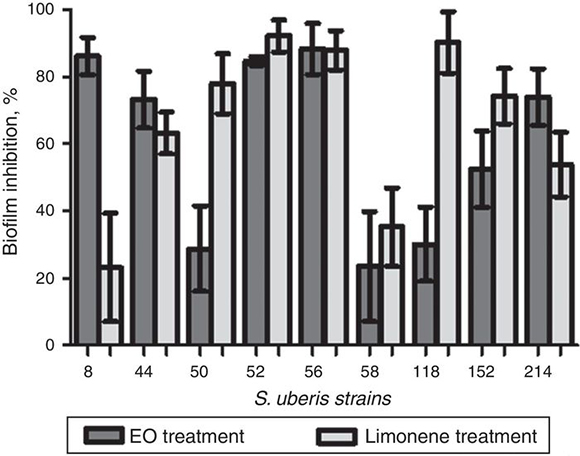
Figure 2. Effect of minimum inhibitory concentrations (MICs) of essential oils and limonene against biofilm of S. uberis.
Different studies have evaluated the efficacy of plant essential oils for improving milk quality in dairy cattle. The literature reports numerous studies referring to the antibacterial activity of essential oils against other pathogens isolated from bovine mastitis such as Staphylococcus spp., S. aureus, Streptococcus agalactiae, Bacillus cereus, E. coli1,9,25. However, few studies demonstrated the antibacterial activity of essential oils against S. uberis. Lambrecht Gonçalves et al.17, evaluated the antibacterial activity of the essential oils of Cymbopogon citratus (DC.) Stapf., Elionurus sp. and Tagetes minuta L., against bacteria isolated from bovine milk. These authors obtained MIC values of 0.9%, 0.15% and 0.75% v/v, respectively, for the three vegetal species against S. uberis, which were similar to those obtained with limonene in our study.
Mullen et al.22 evaluated the antibacterial activity of Phyto-Mast (Bovinity Health LLC, Narvon, PA), an herbal intramammary product, against 3 mastitis-causing pathogens: S. aureus, S. chromogenes, and S. uberis by a modified protocol for broth dilution in vitro. The presence of the essential oil of Thymus vulgaris (thyme) in the formula may account for antibacterial action. The results showed that thyme essential oil had consistent antibacterial activity against the 3 mastitis-causing organisms tested.
The effect of EO and limonene against biofilm formation in S. uberis has not been previously reported. However, different studies showed the inhibition of S. aureus biofilm formation by essential oils. Aiemsaard et al.1 determined the effects of lemongrass oil in inhibiting biofilm formation of S. aureus isolated from bovine mastitis and they found that, it had 44.9 ± 7.4%, inhibition on S. aureus biofilm formation at concentrations of 0.025% v/v of lemongrass oil.
It is important to note that the toxic effect of M. verticillata EO and its main compounds has been evaluated in vitro5,7,12,32 and in vivo5,7,12,13,32. These authors have found no toxic effects of M. verticillata EO and its main compounds.
In conclusion, this study demonstrated that M. verticillata EO, as well as limonene, one of its main components showed antimicrobial efficacy against S. uberis strains presented as alternatives to be evaluated in vivo for the treatment of intramammary infections caused by this agent in cattle. However, further studies are needed to determine the mode of action of EO and limonene.
These results reinforce the importance of compounds isolated from plants and their influence on the elimination of pathogenic microorganisms, reaffirming the role of natural products.
Ethical disclosures
Protection of human and animal subjects
The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data
The authors declare that no patient data appear in this article.
Right to privacy and informed consent
The authors declare that no patient data appear in this article.
Conflict of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
This work was supported by grants from Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina (CONICET) and PICT2268/13. Mic. Ivana Dalila Montironi has Fellowship from CONICET. Dr. Laura Noelia Cariddi and Dr. Elina Beatriz Reinoso are Members of the Research Career of CONICET.
1. Aiemsaard J, Aiumlamai S, Aromdee C, Taweechaisupapong S, Khunkitti W. The effect of lemongrass oil and its major components on clinical isolate mastitis pathogens and their mechanisms of action on Staphylococcus aureus DMST 4745. Res Vet Sci. 2011;91:31-7. [ Links ]
2. Bluma R, Amaiden MR, Daghero J, Etcheverry M. Control of Aspergillus section Flavi growth and aflatoxin accumulation by plant essential oils. J Appl Microbiol. 2008;105:203-14. [ Links ]
3. Cariddi LN, Panero A, Demo MS, Grosso M, Zygadlo J, Sabini LI, Maldonado AM. Inhibition of immediate-type allergic reaction by Minthotachys verticillata (Griseb.) Epling essential oil. J Essent Oil Res. 2007;19:190-6. [ Links ]
4. Cariddi L, Moser M, Andrada MC, Demo MS, Zygadlo JA, Sabini LI, Maldonado AM. The effect of Minthostachys verticillata essential oil on the immune response of patients allergic to dust mites. BLACPMA. 2009;8:224-33. [ Links ]
5. Cariddi L, Escobar F, Moser M, Panero A, Alaniz F, Zygadlo J, Sabini L, Maldonado A. Monoterpenes isolated from Minthostachys verticillata (Griseb.) Epling essential oil modulates immediate-type hypersensitivity responses in vitro and in vivo. Planta Med. 2011;77:1687-94. [ Links ]
6. Cariddi L, Montironi I, Reinoso E. Evaluación de la actividad antimicrobiana del aceite esencial de Minthostachys verticillata y uno de sus compuestos mayoritarios sobre cepas aisladas de mastitis bovina. Dominguezia. 2013;29:96. [ Links ]
7. Cariddi LN. The role of essential oil of Minthostachys verticillata (Griseb) Epling, (Lamiaceae) and its active metabolites in immediate-type hypersensitivity responses. In: Govil JN, Bhattacharya S, editors. Recent progress in medicinal plants essential oils II. Houston, USA: Studium Press, LLC; 2013. p. 487-95. [ Links ]
8. Clinical and Laboratory Standards Institute, Third Edition Document, M31-A3, Wayne, PA, US Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; approved standard; 2008.
9. Dal Pozzo M, Santurio DF, Rossatto L, Vargas AC, Alves SH, Loreto ES, Viegas J. Activity of essential oils from spices against Staphylococcus spp. isolated from bovine mastitis. Arq Bras Med Vet Zootec. 2011;63:1229-32. [ Links ]
10. De Feo V. Medicinal and magical plants in the northern Peruvian Andes. Fitoterapia. 1992;63:417-40. [ Links ]
11. De Feo V, Ricciardi A, Biscardi D, Senatore F. Chemical composition and antimicrobial screening of the essential oil of Minthostachys verticillata (Griseb.) Epling. Lamiaceae. J Essent Oil Res. 1998;10:61-5. [ Links ]
12. Escobar FM, Cariddi LN, Sabini MC, Reinoso E, Sutil SB, Torres CV, Zanon SM, Sabini LI. Lack of cytotoxic and genotoxic effects of Minthostachys verticillata essential oil: studies in vitro and in vivo. Food Chem Toxicol. 2012;50:3062-7. [ Links ]
13. Escobar FM, Sabini MC, Cariddi LN, Sabini LI, Mañas F, Cristofolini A, Bagnis G, Gallucci MN, Cavaglieri RL. Safety assessment of essential oil from Minthostachys verticillata (Griseb.) Epling (peperina): 90-day oral subchronic toxicity study in rats. Regul Toxicol Pharmacol. 2015;71:1-7. [ Links ]
14. González MJ, Marioli JM. Antibacterial activity of water extracts and essential oils of various aromatic plants against Paenibacillus larvae, the causative agent of American Foulbrood. J Invertebr Pathol. 2010;104:209-13. [ Links ]
15. Jayarao B, Dore J, Oliver S. Restriction fragment length polymorphism analysis of 16S ribosomal DNA of Streptococcus and Enterococcus species of bovine origin. J Clin Microbiol. 1992;30:2235-40. [ Links ]
16. Khan I, Hassan A, Abdulmawjood A, Lämmler C, Wolter W, Zschöck M. Identification and epidemiological characterization of Streptococcus uberis isolated from bovine mastitis using conventional and molecular methods. J Vet Sci. 2003;4: 213-23. [ Links ]
17. Lambrecht Gonçalves C, Bender Almeida Schiavon D, Voigt Mota F, Faccin A, Noremberg Schubert R, Schiedeck G, Damé Schuch LF. Actividad antibacteriana de los extractos de Cymbopogon citratus, Elionurus sp. y Tagetes minuta contra bacterias que causan mastitis. Rev Cubana Plant Med. 2013;18:487-94. [ Links ]
18. Larriestra A, Vissio C. Mastitis: pérdidas económicas, frecuencia y variantes de la enfermedad. ¿Cuántos $$ se lleva la mastitis? Sitio argentino de producción nacional. Producir XXI, Bs As. 2012;20:28-32. [ Links ]
19. Li H, Yang T, Li F-Y, Yao Y, Sun Z-M. Antibacterial activity and mechanism of action of Monarda punctata essential oil and its main components against common bacterial pathogens in respiratory tract. Int J Clin Exp Pathol. 2014;7:7389-98. [ Links ]
20. Maldonado AM, Calvo D, Cariddi LN, Demo M. Efectos linfoproliferativos y antimicrobianos de productos vegetales derivados de Minthostachys verticillata y Achyrocline satureioides. Archivos de Alergia e Inmunología Clínica. 2001;32:82. [ Links ]
21. Malinowski E. The use of some immunomodulators and nonantibiotic drugs in a prophylaxis and treatment of mastitis. Pol J Vet Sci. 2002;5:197-202. [ Links ]
22. Mullen KAE, Lee AR, Lyman RL, Mason SE, Washburn SP, Anderson KL. Short communication: an in vitro assessment of the antibacterial activity of plant-derived oils. J Dairy Sci. 2014;97:5587-91. [ Links ]
23. Nunes ELC, Barbosa EV, Folly E, Lione VF, Castro HC. Bovine mastitis: a brief reminder about a potential target for exploring medicinal plants use. IJMPAM. 2013;1:80-6. [ Links ]
24. Ojeda M, Coirini R, Cosiansi J, Zapata R, Zygadlo J. Evaluation of variability in natural populations of peperina (Minthostachys mollis (Kunth.) Griseb.), an aromatic species from Argentina. Plant Genet Resour Newsl. 2001;126: 27-30. [ Links ]
25. Perini S, Piccoli RH, Nunes CA, Bruhn FRP, Custódio DAC, Costa GM. Antimicrobial activity of essential oils against pathogens isolated from Bovine Mastitis. J Nat Prod Plant Resour. 2014;4:6-15. [ Links ]
26. Primo V, Rovera M, Zanon S, Oliva M, Demo M, Daghero J, Sabini L. Determination of the antibacterial and antiviral activity of the essential oil from Minthostachys verticillata (Griseb.) Epling. Rev Argent Microbiol. 2001;33:113-7. [ Links ]
27. Pyörälä S. New strategies to prevent mastitis. Reprod Dom Anim. 2002;37:211-6. [ Links ]
28. Reinoso E, Fambrini A, Lasagno M, Odierno L. Biofilm production by strains isolated from bovine mastitis. Biocell. 2011; 35:118. [ Links ]
29. Schmidt-Lebuhn AN. Ethnobotany, biochemistry and pharmacology of Minthostachys (Lamiaceae). J Ethnopharmacol. 2008;118:343-53. [ Links ]
30. Senatore F. Volatile constituentes of Minthostachys setosa (Briq.) Epling. (Lamiaceae) from Perú. Flav Frag J. 1998;13:263-5. [ Links ]
31. Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods. 2000;40:175-9. [ Links ]
32. Sutil SB, Astesano A, Vogt V, Torres CV, Zanon SM, Sabini LI. Minthostachys verticillata: toxicity of its essential oil and major constituents to Artemia salina and cell lines. Mol Med Chem. 2006;10:41-2. [ Links ]
33. Varhimo E, Pekka V, Fallarero A, Skogman M, Pyörälä S, Iivanainen A, Sukura A, Vuorela P, Alpha-and Savijoki K. β-casein components of host milk induce biofilm formation in the mastitis bacterium Streptococcus uberis. Vet Microbiol. 2011;49:381-9.
34. Zygadlo J, Maestri D, Lamarque A, Guzmán C, Velasco- Negueruela A, Pérez-Alonso M, García-Vallejos M, Grosso N. Essential oil variability of Minthostachys verticillata. Biochem Syst Ecol. 1996;24:319-23. [ Links ]














