Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista argentina de microbiología
versión impresa ISSN 0325-7541versión On-line ISSN 1851-7617
Rev. argent. microbiol. vol.49 no.1 Ciudad Autónoma de Buenos Aires mar. 2017
http://dx.doi.org/10.1016/j.ram.2016.09.008
ORIGINAL ARTICLE
http://dx.doi.org/10.1016/j.ram.2016.09.008
Efficacy of epiphytic bacteria to prevent northern leaf blight caused by Exserohilum turcicum in maize
Eficacia de bacterias epifíticas en la prevención del tizón foliar del maíz causado por Exserohilum turcicum
Melina Sartoria, Andrea Nescia, Julián Garcíab, María A. Passonea, Analía Montemarania, Miriam Etcheverrya,*
a. Laboratorio de Ecología Microbiana, Departamento de Microbiología e Inmunología, Facultad de Ciencias Exactas Físico Químicas y Naturales, Universidad Nacional de Río Cuarto, Río Cuarto, Córdoba, Argentina
b. Oro Verde, Servicios fitosanitarios, Río Cuarto, Córdoba, Argentina
Received 11 July 2015; accepted 13 September 2016
Available online 8 February 2017
*Corresponding author.
E-mail address: metcheverry@exa.unrc.edu.ar (M. Etcheverry).
0325-7541/© 2016 Asociación Argentina de Microbiología. Published by Elsevier Espana, S.L.U. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Abstract
Eight potential biological control agents (BCAs) were evaluated in planta in order to assess their effectiveness in reducing disease severity of northern leaf blight caused by Exserohilum turcicum. The assay was carried out in greenhouse. Twenty-six-day-old plants, V4 phenological stage, were inoculated with antagonists by foliar spray. Only one biocontrol agent was used per treatment. Ten days after this procedure, all treatments were inoculated with E. turcicum by foliar application. Treatments performed were: C-Et: control of E. turcicum; T1: isolate 1 (Enterococcus genus) + E. turcicum; T2: isolate 2 (Corynebacterium genus) + E. turcicum; T3: isolate 3 (Pantoea genus) + E. turcicum; T4: isolate 4 (Corynebacterium genus) + E. turcicum; T5: isolate 5 (Pantoea genus) + E. turcicum; T6: isolate 6 (Bacillus genus) + E. turcicum; T7: isolate 7 (Bacillus genus) + E. turcicum; T8: isolate 8 (Bacillus genus) + E. turcicum. Monitoring of antagonists on the phyllosphere was performed at different times. Furthermore, the percentage of infected leaves and, plant and leaf incidence were determined. Foliar application of different bacteria significantly reduced the leaf blight between 30-78% and 39-56% at 20 and 39 days respectively. It was observed that in the V10 stage of maize plants, isolate 8 (Bacillus spp.) caused the greatest effect on reducing the severity of northern leaf blight. Moreover, isolate 8 was the potential BCA that showed more stability in the phyllosphere. At 39 days, all potential biocontrol agents had a significant effect on controlling the disease caused by E. turcicum.
Keywords
Exserohilum turcicum; Biological control; Prevention strategy; Phyllospheric bacteria.
Resumen
Se evaluó a 8 potenciales agentes de control biológico (ACB) en un ensayo in planta, con el objetivo de probar su efectividad en la reducción del daño provocado por Exserohilum turcicum, agente causal del tizón foliar del maíz. El ensayo se llevó a cabo en invernadero. Plantas de maíz de 26 días, en estadio fenológico V4, se inocularon con los potenciales antagonistas por aplicación foliar como espray. Solo un agente de biocontrol fue usado por tratamiento y todos los tratamientos se inocularon con E. turcicum 10 días después, también por aplicación foliar. Los tratamientos desarrollados fueron los siguientes: C-Et: control de E. turcicum; T1: aislamiento 1 (género Enterococcus) + E. turcicum; T2: aislamiento 2 (género Corynebacterium) + E. turcicum; T3: aislamiento 3 (género Pantoea) + E. turcicum; T4: aislamiento 4 (género Corynebacterium) + E. turcicum; T5: aislamiento 5 (género Pantoea) + E. turcicum; T6: aislamiento 6 (género Bacillus) + E. turcicum; T7: aislamiento 7 (género Bacillus) + E. turcicum; T8: aislamiento 8 (género Bacillus) + E. turcicum. La monitorización en la filosfera de los antagonistas se llevó a cabo a diferentes tiempos. Además, se determinó el porcentaje de hojas infectadas y la incidencia en plantas y hojas. La aplicación foliar de diferentes bacterias redujo significativamente la gravedad del tizón del maíz: entre el 30 y el 78% a los 20 días y entre el 39 y el 56% a los 39 días. En el estadio V10 de las plantas de maíz se observó que el aislamiento 8 (Bacillus spp.) causó el mayor efecto de reducción del tizón foliar. Además, dicho aislamiento fue el potencial agente de biocontrol que mostró mayor estabilidad en la filosfera. A los 39 días, todos los potenciales agentes de biocontrol demostraban un efecto significativo sobre el control de la enfermedad causada por E. turcicum.
Palabras clave
Exserohilum turcicum; Control biológico; Estrategias de prevención; Bacterias filosféricas.
Introduction
Northern leaf blight caused by Exserohilum turcicum (Pass.) Leonard and Suggs (Syn. Helminthosporium turcicum Pass.) is an endemic foliar disease in the maize production area of Argentina7. This pathogen has been known for its high prevalence and intensity in recent seasons in late planting of maize10,21. Several factors have influenced the increase of disease, such as late planting dates, expansion of the area under reduced tillage11 and intense and frequent rainfall during the summer months18,21. E. turcicum is a pathogen characterized by low spore production, long latency periods and lesions with expanded spots17. Disease affects photosynthetic tissues and the increase of lesion size can cause necrosis of the complete leaf. Necrosis and premature leaf death diminishes the capture of solar energy and consequently the translocation of photosynthates necessary for grain filling21. The progress of the disease is favored by moderate temperatures and long leaf-wetness periods due to rain or dew, conditions that commonly occur in the central maize area of Argentina, coinciding with the reproductive stages of the crop15. A 50% reduction of incident radiation 15 days before and 15 days after flowering may cause a decrease of 40-50% of grain yield20. In the central area of Argentina, De Rossi et al.14 have determined that values of 60% severity caused losses of up to 40% in the yield of susceptible hybrids.
The control techniques used so far are chemical control and genetic resistance22. It has been determined that mixtures of triazoles and strobilurins are valid tools for the reduction of yield losses caused by northern leaf blight9,12,14,19,33,40. Moreover, the widespread use of chemicals in agriculture has been a subject of public concern and scrutiny due to possible harmful effects on the environment, their undesirable effects on non-target organisms and possible carcinogenicity of some chemicals27. Therefore, the need to develop non-chemical methods to prevent and/or control plant diseases is clear26. Genetic resistance with utilization of tolerant hybrids is key to maintaining competitiveness in areas where disease is present18,39. The use of selected hybrids must be accompanied by cultural practices avoiding monoculture22. Resistance, however, is labor-intensive to score and can only be measured late in the growing season31.
For these reasons, we believe that there must be a movement to adopt biological control strategies capable of ensuring crop protection. Even more, this protection is achieved when the microorganisms used to antagonize foliar pathogens come from the same ecosystem. Phyllospheric microbiota could contribute to the health of plant species through surface protection against pathogens29. Bacteria having antagonistic activity against southern leaf blight caused by the fungus Cochliobolus heterostrophus (Drechs.) have been shown in maize44. Different non-pathogenic organisms were evaluated as possible antagonists of foliar diseases of crops24,43. The phyllosphere is an environment that is subject to continuous variations in humidity and temperature, exposure to ultraviolet radiation, and limited nutrient availability4. Hence, bacteria must be stable to fluctuating abiotic factors3. Therefore, potential biological control agents require a survival strategy to environmental stresses and maintaining a threshold population on leaf surfaces28.
In a previous study we performed selection steps of possible biological control agents of E. turcicum, taking into consideration ecological parameters relevant to the maize agroecosystem37. Antagonistic interactions in vitro of bacteria with the pathogen were evaluated using competition for nutrients, antibiosis and the reduction effect on growth parameters. Due to the need to assess the detrimental effects of the potential antagonists selected in vitro against E. turcicum in planta, the objectives proposed in this study were: (a) to evaluate the effectiveness of bacterial antagonists in reducing disease severity in the greenhouse assay, and (b) to evaluate the maintenance of potential biocontrol agents in the phyllosphere throughout the assay.
Materials and methods
E. turcicum
The fungal strain of E. turcicum was previously isolated from maize (DK 190) growing on Campus Santa Julia of National University of Córdoba (UNC), in Córdoba province, Argentina. The isolate was maintained at 4 °C on potato dextrose agar medium (PDA: dextrose 20 g, potato extract 4 g, agar 15 g, distilled water 1000 ml, final pH 5.6 ± 0.2) and in 15% glycerol at −80 °C.
E. turcicum isolate was subcultured on PDA plates and incubated at 25 °C for 30 days to enable significant sporulation. The surface of colonies grown on PDA were scraped and diluted in 300 ml of sterile water with vaseline (2%). Inoculum concentration was verified using a Neubauer chamber. An inoculum density of 2 × 104 spores/ml in distilled water plus 0.06 g/l of Triton x-100 was obtained.
Potential biological control agents (BCA)
All antagonistic bacteria were isolated from maize leaves with blight lesions from fields of cultivars in Chucul, Río Cuarto and Vicuña Mackenna, all in Córdoba province, Argentina. The antagonistic ability was previously evaluated in vitro, and eleven potential control agents against E. turcicum were selected37. In this study we evaluated the antagonistic effect of eight of these isolates. Isolate 1 was identified as Enterococcus, isolates 2 and 4 belonged to Corynebacterium, isolates 3 and 5 were included in the Pantoea genus, and finally, isolates 6, 7 and 8 were identified as Bacillus37. These isolates were maintained on slants of trypticase soy agar (TSA). Spontaneous mutants resistant to streptomycin 5% and rifampicin 0.5% were obtained.
Bacterial isolates were cultured on trypticase soy broth (TSB) for 24 h at 140 rpm and 25 °C. Afterwards, serial dilutions were performed and plated on TSA to evaluate cell viability and to determine the number of colony forming units per ml (CFU/ml). Cultures of different antagonists were diluted in TSB to obtain inocula of 108 to 1010 CFU/ml28,42.
Greenhouse assays
The experimental design was completely randomized with three blocks and four replications. Pots of 5 l capacity were prepared with a mixture of fertile soil and perlite to promote aeration and water reserve. Four seeds of maize susceptible to northern leaf blight Dekalb 67013 were sown per pot, that is, twelve plants per treatment were evaluated. Treatments performed were: C-Et: control of E. turcicum; T1: isolate 1 (Enterococcus genus) + E. turcicum; T2: isolate 2 (Corynebacterium genus) + E. turcicum; T3: isolate 3 (Pantoea genus) + E. turcicum; T4: isolate 4 (Corynebacterium genus) + E. turcicum; T5: isolate 5 (Pantoea genus) + E. turcicum; T6: isolate 6 (Bacillus genus) + E. turcicum; T7: isolate 7 (Bacillus genus) + E. turcicum; T8: isolate 8 (Bacillus genus) + E. turcicum. Only one biocontrol agent was used per treatment. Foliar applications with each biocontrol agent alone served as control.
Twenty-six-day-old plants, V4 phenological stage36, were inoculated with antagonists by foliar spray, using an atomizer. Ten days after this procedure, all treatments were inoculated with E. turcicum by foliar application. Plants were covered for 24 h with transparent polyethylene bags simulating a chamber with 100% relative humidity (RH)1, and then kept in a greenhouse with an average temperature of 20 °C. Artificial light was applied when necessary. Pots were watered daily or as required.
Monitoring of BCA in the phyllosphere
The survival of antagonists on the phylloplane of maize was determined using antibiotic resistance as marker28. Monitoring of antagonists on the phyllosphere was performed at time of inoculation (time 0), 48 h post-inoculation (time 1), 24 h after inoculation of pathogen (time 2) and 39 days post-inoculation of antagonists (time 3). A leaf from each pot was weighed and suspended in phosphate buffer to obtain a 1:10 dilution and incubated for 1 h at 180 rpm and 30 °C. Afterwards, serial dilutions were performed in nutrient broth (NB) and plated on nutrient agar (NA) with rifampicin for isolates 3 and 5, and with streptomycin for isolates 1, 2 and 4. A medium without antibiotic was used for isolates 6, 7 and 8, since they were susceptible to them. Plates were incubated for 48 h at 30 °C, and then CFU counts were performed. To analyze the variations of isolates on the phyllosphere, principal component analysis (PCA) with the same classification variable and time was performed2. The PCA and biplot graphics are known as generally used techniques for interpreting reductions. Artificial axes of PCA (principal components) plotted observations and/or variables of the optimal properties for the interpretation of the underlying variability and co-variability. The CFU variable of different treatments and different times was analyzed. A scatter plot was obtained with PCA, representing treatment and time observations.
Assessment of disease severity
Fifteen days after inoculation of the pathogen and up to 39 days, the effect of treatments on disease was analyzed by determining the percentage of leaf tissue infected with E. turcicum using the scale developed by Bleicher5. This scale measured on four levels (0: undeveloped, 1: incipient development with lesions lower than 5 cm, 2: medium development with lesions larger than 5 cm, 3: advanced development in most parts of the leaf) allows us to evaluate severity until day 40 post-inoculation.
Furthermore, the impact on plant (percentage of affected plants relative to total plants of the treatment)23 and incidence in leaves (percentage of leaves with at least one lesion relative to total leaves)34 were evaluated. Severity of foliar blight until VT phenological stage (39 days post-inoculation) was statistically analyzed with ANOVA, InfoStat 2013. PCA was also performed with treatments as classification variable2,16.
Results
Figure 1 shows the severity of leaf blight in the different treatments evaluated during 39 days after pathogen inoculation. Severity in control treatment varied from 2.1 to 51.5% during 15 to 39 days after pathogen application. Foliar application of different bacteria significantly reduced the leaf blight between 30-78% and 39-56% at 20 and 39 days respectively.
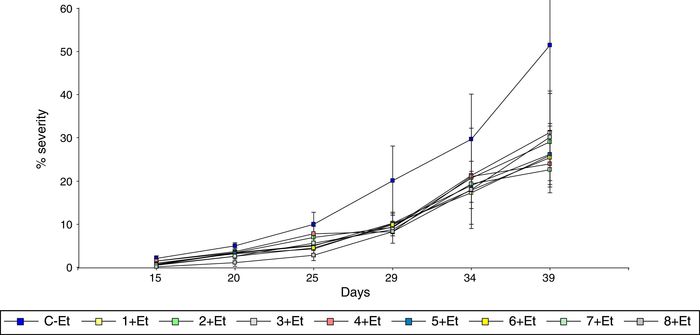
Figure 1 Evaluation of different bacteria for control of foliar blight of maize. Disease was evaluated during 39 days as percentage of leaf area. Data points are the mean values and standard deviation of 12 plants (average of affected foliar area in plants of three blocks) for each treatment. C-Et: control of E. turcicum; 1 + Et: isolate 1 (Enterococcus genus) + E. turcicum; 2 + Et: isolate 2 (Corynebacterium genus) + E. turcicum; 3 + Et: isolate 3 (Pantoea genus) + E. turcicum; 4 + Et: isolate 4 (Corynebacterium genus) + E. turcicum; 5 + Et: isolate 5 (Pantoea genus) + E. turcicum; 6 + Et: isolate 6 (Bacillus genus) + E. turcicum; 7 + Et: isolate 7 (Bacillus genus) + E. turcicum; 8 + Et: isolate 8 (Bacillus genus) + E. turcicum.
The sum of two principal components (CP1 and CP2) explained 94.6% of the total data variability (Fig. 2). It was observed that isolate 8 showed the greatest effect on reducing the severity of northern leaf blight showing a negative correlation (angles between vectors over 90°) with respect to control treatment (C-Et). Other treatments that showed a significant reduction effect were those using isolates 6, 7 and another group of treatments with isolates 5, 1, and 3. In terms of severity progress, a positive correlation was observed over time. Until day 25 post-inoculation a similar effect was observed. However, as from day 29 the greatest effect on severity was manifested.

Figure 2 Principal component analysis of different treatments and effect on severity of leaf blight. Variables analyzed: treatments and days. C-Et: control of E. turcicum; 1 + Et: isolate 1 (Enterococcus genus) + E. turcicum; 2 + Et: isolate 2 (Corynebacterium genus) + E. turcicum; 3 + Et: isolate 3 (Pantoea genus) + E. turcicum; 4 + Et: isolate 4 (Corynebacterium genus) + E. turcicum; 5 + Et: isolate 5 (Pantoea genus) + E. turcicum; 6 + Et: isolate 6 (Bacillus genus) + E. turcicum; 7 + Et: isolate 7 (Bacillus genus) + E. turcicum; 8 + Et: isolate 8 (Bacillus genus) + E. turcicum.
Table 1 shows mean leaf blight incidence and severity using different possible biocontrol agents in the middle and at the end of the trials under greenhouse conditions. Data showed that the highest mean severity was recorded in the control treatment at the two times evaluated, whereas the lowest was found in the treatment with isolate 8 at 20 days and with all possible biocontrol agents at 39 days, showing a range of 40-56% of severity reduction.
Table 1 Mean leaf blight incidence and severity with different treatments under greenhouse conditions at middle (20 days post-inoculation) and end (39 days post-inoculation) of trials.

Plants incidence: proportion of disease plants of 12 total plants.Leaves incidence: average value of affected leaves of each plant.Severity: average value of diseased tissue area of 12 total plants.Number of lesions: lesions per plant.Values followed by different letters within a column indicate significant differences between treatments at p < 0.10 for each day of post-inoculation and each parameter determined according to DGC test. C-Et: control of E. turcicum; 1 + Et: isolate 1 (Enterococcus genus) + E. turcicum; 2 + Et: isolate 2 (Corynebacterium genus) + E. turcicum; 3 + Et: isolate 3 (Pantoea genus) + E. turcicum; 4 + Et: isolate 4 (Corynebacterium genus) + E. turcicum; 5 + Et: isolate 5 (Pantoea genus) + E. turcicum; 6 + Et: isolate 6 (Bacillus genus) + E. turcicum; 7 + Et: isolate 7 (Bacillus genus) + E. turcicum; 8 + Et: isolate 8 (Bacillus genus) + E. turcicum.
Isolate 8 significantly reduced plant incidence (27%) at 20 days, but this effect was not maintained until the end of the assay. An increase of leaf incidence and number of lesions for control and treatments with possible biocontrol agents was observed between 20 and 39 days.
All possible biocontrol agents were able to maintain high population sizes in the phyllosphere during time 0 to time 3 (Table 2). Population counts were found in the range of 9.04-4.88 log. Bacillus isolates reduced their count only one log between time 0 to time 1, and this count (6 log) was maintained until time 3. The population count of Enterococcus and one isolate of Corynebacterium was reduced by 3 log, while Pantoea and another isolate of Corynebacterium showed a 5-log reduction at the end of the assay.
Table 2 Survival of antagonists (Log CFU/g) onto maize phylloplane at different time.

Time 0: time of inoculation; Time 1: 48 h post-inoculation; Time 2: 20 days post-inoculation; Time 3: 39 days post-inoculation; Antagonists: 1: Enterococcus spp.; 2: Corynebacterium spp.; 3: Pantoea spp.; 4: Corynebacterium spp.; 5: Pantoea spp.; 6: Bacillus spp.; 7: Bacillus spp.; 8: Bacillus spp.
The sum of two principal components, for analyzing the maintenance of bacterial population in the phyllosphere, was 89.2% (Fig. 3). Data showed that the bacterial population of isolates 1, 3 and 5 decreased through time. Bacterial isolate 8 was able to maintain its population size more stable, followed by isolates 4 and 7.
 Ç
Ç
Figure 3 Principal component analysis of maintenance of possible biocontrol agents in phyllosphere. Variables analyzed: isolate (C) and time. Time 1 (T1): 48 h post-inoculation; time 2 (T2): 20 days; time 3 (T3): 39 days; C1: Enterococcus spp.; C2: Corynebacterium spp.; C3: Pantoea spp.; C4: Corynebacterium spp.; C5: Pantoea spp.; C6: Bacillus spp.; C7: Bacillus spp.; C8: Bacillus spp.
A general correlation analysis of the antagonist population and severity of disease at day 39 post-inoculation showed a negative correlation (r −0.08), not statistically significant (p 0.726).
Discussion
The foliar application of possible biocontrol agents to maize leaves led to the effective control of northern leaf blight caused by E. turcicum under greenhouse conditions. In this study, the plants treated with any antagonist significantly decreased the severity of the symptoms caused by the pathogen at the end of the assay.
Isolates belonging to the Bacillus genus were the most effective in reducing the severity of disease and also the incidence in plants and the number of lesions at 20 days. At 39 days, all potential biocontrol agents showed better effect on severity reduction, obtaining 40 to 56% reduction in this parameter.
Stromberg et al.42 demonstrated that the reduction in pathogen population density by antagonists was correlated with the reduction in intensity of foliar disease, and therefore biological control was described as pre-symptomatic reduction in pathogen population size caused by antagonists, and not as a result of induced resistance or changes in the odds of cell infection by the pathogen. Consistently, several studies have demonstrated an inhibitory effect of different antagonists against E. turcicum due to competition and/or antibiosis25,30,35. In a previous screening study in vitro, isolates belonging to the Bacillus genus (6, 7, and 8) showed dominance of the pathogen at a distance and a reduction of E. turcicum growth rate of between 84 and 98%, indicating that it may have an ability to synthesize a diffusible substance with inhibitory capacity. Moreover, a significant negative correlation was observed between E. turcicum growth rate and dominance index when the pathogen interacted with selected bacteria37. In the present study, isolate 8 showed the highest effect on reducing northern leaf blight in maize plants at the V6 phenological stage, followed by isolates 6 and 7.
Corynebacterium spp. (isolate 4) and 3 strains of the Bacillus genus reached a 6-log population size at 39 days, with Bacillus isolates having the highest population survival. Moreover coincidentally, isolate 8 was the potential biological control agent that showed more stability on the phyllosphere, showing minor variations in the population count at the end of the trial. Evidently, high bacteria count can allow for competitive exclusion of certain species32. It is noteworthy that all analyzed BCAs maintained high population levels at day 39. Considering that bacteria were not physiologically adapted, a necessary process to increase their tolerance to fluctuating conditions of the environment38, the maintenance of these bacteria in phyllosphere may be by their condition of native bacteria. Beattie and Lindow3 argue that phyllobacteria can modify their environment to enhance their colonization of plants by increasing local nutrient concentrations or by producing a layer of extracellular polysaccharides either in the surface of leaves or in the interior.
We propose the use of biological control agents as a strategy to prevent the incidence of E. turcicum in maize. Although BCAs have a different mode of action to chemical agents, a biological strategy may favor the control of the pathogen performing a second application in V9 and V10 stages included in the critical period of the crop. The critical period of the crop to control this disease extends from V8 (eight leaves unfolded) to R3 (aqueous grain). However, the stage where the probability of response to the application of fungicides is higher, ranging from 15 days before to 15 days after silking (start of panicle or VT and the development of whiskers or R1)8. In general, the application threshold of chemical agents for common blight corresponds to one lesion less or equal to 1 cm long on each sheet6. In this study, all possible biocontrol agents used were able to reduce severity between 40 to 56% in V10. Therefore, we suggest a second application of BCAs in critical periods. Thinking about the use of biological control agents as a strategy for prevention of disease caused by E. turcicum in maize, the application was done in plants of 26 days of development (V 4), that is 10 days before the application of the pathogen. Despite the fact that this preventive treatment was effective in reducing severity by between 40 and 56% at the end of the trial, the average leaf affected was greater than 1 in all treatments. Probably, a second application of BCAs in the critical period could reduce the average of affected leaves, plant incidence and number of lesions. Sillon et al.41 showed 100% incidence of E. turcicum in plants of resistant hybrids in Santa Fe, Argentina; however, severity in leaves did not exceed 20%.
It is known that yield losses are greater when infection occurs early and moves to the upper leaves of the plant or on grain filling (R1 to R3). Therefore the slow progress of the disease in relation to crop development reduces the impact of disease. Formento21 has pointed out that if the infection is delayed until six weeks after stigma fertilization, yield reduction will be minimal compared to an infection that occurs before fertilization.
The physiological adaptation of bacteria is being carried out in order to facilitate their maintenance in the phyllosphere. In addition, different inoculum doses of previously selected antagonists, as well as the number and period of BCA applications will be determined in order to achieve control or delay the development of leaf blight during the critical period of maize growth.
Ethical disclosures
Protection of human and animal subjects
The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data
The authors declare that no patient data appear in this article.
Right to privacy and informed consent
The authors declare that no patient data appear in this article.
Conflict of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
This work was carried out through a grant from the Agencia Nacional de Promoción Científica y Tecnológica, PICT 2268/12 and SECYT-UNRC. 2010-2013 - Res. 1001/12 annual Res. 361/13.
1. Aden MH [M.Sc. thesis] Studies of sorghum leaf blight incited by Exserohilum turcicum (Pass.) Leo. & Suggs. Rajendranagar: Faculty of Agriculture, Andra Pradesh Agricultural University; 1991.
2. Balzarini MG, Gonzalez L, Tablada M, Casanoves F, Di Rienzo JA, Robledo CW. Infostat. Manual Del Usuario. Córdoba, Argentina: Editorial Brujas; 2008. [ Links ]
3. Beattie G, Lindow S. Bacterial colonization of leaves: a spectrum of strategies. Phytopathology. 1999;89:353-9. [ Links ]
4. Blakeman JP. Phylloplane interactions. In: Mount MS, Lacy GH, editors. Phytopathogenic prokaryotes, vol. 120a1. 1982. p. 307-33.
5. Blakeman JP, Fokkeman NJ. Potential for biological control of plant diseases on the phylloplane. Annual Review of Phytopathology. 1982;20:167-92. [ Links ]
6. Carmona M. Umbrales para el control de las enfermedades foliares del maíz 2014; 2014. Available from: http://www.agroconsultasonline.com.ar/documento.html. [ Links ]
7. Carmona M, Reis E, Gally M. Pudriciones de tallo y raíces en el cultivo de maíz. Revista maíz en siembra directa, AAPRESID; 2006. p. 86-9. [ Links ]
8. Consagro Maíz de primera. Impacto de las enfermedades de hoja en lotes de productores durante la campaña 2014-2015; 2014. Available from: www.consagro.com.ar/informes-tecnicos/090115-maiz-de-primera/. [ Links ]
9. Couretot L, Parisi L, Ferraris G, Magnone G. Efecto de fungicidas foliares y momento de aplicación sobre la severidad de tizón foliar y enfermedades de raíz y tallo en maíz. In: XIV Jornadas Fitosanitarias Argentinas. 2012. [ Links ]
10. Couretot L. Principales enfermedades del cultivo de maíz. In: VI Jornada de Actualización Técnica de Maíz. 2011. [ Links ]
11. Couretot L. Principales enfermedades del cultivo de maíz en la zona Norte de la Prov. de Bs. As. Campana 2009/10; 2010. Available from: http://www.inta.gov.ar/pergamino. [ Links ]
12. Da Costa DI, Boller W. Aerial and ground applications of fungicide for the control of leaf diseases in maize crop (Zea mays L.). In: CIGR - International Conference of Agric. Engineering. 2008. [ Links ]
13. Dekalb Guía de maíces tardíos y de segunda; 2013. Available from: www.dekalb.com.ar. [ Links ]
14. De Rossi R, Plazas M, Brucher E, Ducasse D, Guerra G. El Tizón del Maíz (Exserohilum turcicum): presencia e impacto en el centro norte de Córdoba durante tres campañas agrícolas. In: IX Congreso Nacional de Maíz. 2010.
15. de Souza J. Enfermedades del maíz en Entre Ríos. Actualización Técnica Maíz, Girasol y Sorgo. INTA EEA Paraná Ser Ext. 2007;44:80-5. [ Links ]
16. Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW. InfoStat versión 2013. Argentina: Grupo InfoStat, FCA, Universidad Nacional de Córdoba; 2013 http://www.infostat.com.ar. [ Links ]
17. Díaz C, De Rossi R, Couretot L, Sillon M, Formento N, González V. Prevalencia y distribución de enfermedades del maíz en Argentina. In: 29° Congresso Nacional de Milho e Sorgo. 2012. [ Links ]
18. Díaz C. Evolución e impacto de enfermedades foliares en el cultivo de maíz: Cercospora y Tizones. In: IX Congreso Nacional de Maíz. 2010. p. 200-4. [ Links ]
19. Fantin G, Duarte A. Manejo de doenças na cultura do milho safrinha. Camoinas, Brazil: Instituto Agronomico; 2009. p. 98. [ Links ]
20. Fischer K, Palmer A. Tropical maize. In: Goldsworthy P, Fisher N, editors. The physiology of tropical field crops. New York, USA: Wiley & Sons; 1984. p. 213-48. [ Links ]
21. Formento A. Enfermedades foliares reemergentes del cultivo de maíz: Royas (Puccinia soghi y Puccinia polysora), Tizón foliar (Exserohilum turcicum) y Mancha ocular (Kabatiella zeae). INTA Paraná; 2010. Available from: www.inta.gov.ar/parana/info/documentos/producciónvegetal/maiz/enfermedad. [ Links ]
22. Formento A, Vicentín I. Mancha ocular en maíz (Aureobasidium zeae Syn. Kabatiella zeae); 2005. Available from: www.inta.gov.ar/parana/info/documentos/producciónvegetal. [ Links ]
23. Gautam P, Stein J. Induction of systemic acquired resistance to Puccinia sorghi in corn. Int J Plant Pathol. 2011;2:43-50. [ Links ]
24. Gomes R, Semedo L, Soares R, Linhares L, Ulhoa C, Alviano C. Purification of a thermostable endochitinase from Streptomyces RC1071 isolated from a cerrado soil and its antagonism against phytopathogenic fungi. J Appl Microbiol. 2001;90: 653-61. [ Links ]
25. Harlapur SI, Kulkarni MS, Wali MC, Kulkarni S. Evaluation of plant extract, bio-agents and fungicides against Exserohilum turcicum (Pass.) Leonard and Suggs. causing turcicum leaf blight of maize. Karnataka J Agric Sci. 2007;20:541-4. [ Links ]
26. Heydari A, Pessarakli M. A review in biological control of fungal plant pathogens using microbial antagonists. J Biol Sci. 2010;10:273-90. [ Links ]
27. Heydari A. In: Pessarakli M, editor. Biological control of turfgrass fungal diseases. Trigrams management and physiology. FL, USA: CRC Press; 2007. [ Links ]
28. Kishore GK, Pande S, Podile A. Biological control of late leaf spot of peanut (Arachis hypogaea) with chitinolytic bacteria. Biol Control. 2005;95:1157-60. [ Links ]
29. Lindow S, Brandl M. Microbiology of the phyllosphere. Appl Environ Microbiol. 2003;69:1875-83. [ Links ]
30. Mahamood A, Javed N, Ahmad R, Raheel Z. Biological control of maize leaf blight caused by Helminthosporium turcicum Pass in vitro. Pak J Phytopathol. 1995;7:62-4. [ Links ]
31. Manching H, Balint-Kurti P, Stapleton A. Southern leaf blight disease severity is correlated with decreased maize leaf epiphytic bacterial species richness and the phyllosphere bacterial diversity decline is enhanced by nitrogen fertilization. Front Plant Sci. 2014;5:1-8. [ Links ]
32. Meyer K, Leveau J. Microbiology of the phyllosphere: a playground for testing ecological concepts. Oecologia. 2012;168:621-9. [ Links ]
33. Oddino C, Marinelli A, García J, Garcia M, Tarditi L, Ferrari S, D'Eramo L, March GJ. Comparación del efecto de momentos de tratamientos fungicidas sobre enfermedades foliares del maíz a través de modelos epidemiológicos no flexibles. In: IX Congreso Nacional de Maíz. 2010. [ Links ]
34. Peterson R, Campbell F, Hannah A. A diagrammatic scale for estimating rust intensity on leaves and stems of cereals. Can J Res. 1948;26:496-500. [ Links ]
35. Ramachandra CG [M.Sc. thesis, M.Sc.] Studies on leaf blight of dicoccum wheat caused by Exserohilum hawaiienesis (Bugnicourt). India: University of Agricultural Sciences; 2000.
36. Ritchie S, Hanway J. How a corn plant develops. Ames, Iowa (Special Report N°48): Iowa State University of Science and Technology, Cooperative Extension Service; 1982. [ Links ]
37. Sartori M, Nesci A, Formento A, Etcheverry M. Selection of potential biological control of Exserohilum turcicum with epiphytic microorganisms from maize. Rev Argent Microbiol. 2015;47:62-71. [ Links ]
38. Sartori M, Nesci A, Etcheverry M. Impact of osmotic/matric stress and heat shock on environmental tolerance induction of bacterial biocontrol agents against Fusarium verticillioides. Res Microbiol. 2010;161:681-6. [ Links ]
39. Silloín M. Caracterizacioín y control quiímico de las enfermedades foliares en el cultivo de maíz: tendencias en el ciclo agriícola 2011/2012. Revista Agromercado, Cuadernillo Maiíz. 2012. [ Links ]
40. Sillon M, Ramos J, Del Valle E, Couretot L, Fontanetto H. Nuevos desafíos en maíz: tizones, PTR y nematodos. In: XVIII Congreso de Aapresid. 2010. p. 1-6. [ Links ]
41. Sillon M, Berardo M, Mandrile M, Albrecht J, Fontanetto H, Marinone D. Las enfermedades fúngicas del cultivo de maíz en Santa Fe durante el ciclo agrícola 2008/2009; 2009. Agromercado Clásico N° 157, Maíz, Argentina. [ Links ]
42. Stromberg K, Kinkel L, Leonard K. Quantifying the effect of bacterial antagonists on the relationship between phyllosphere population sizes of Xanthomonas translucens pv. translucens and subsequent bacterial leaf streak severity on wheat seedlings. Biol Control. 2004;29:58-65. [ Links ]
43. Urquhart E, Punja Z. Hydrolytic enzymes and antifungal compounds produced by Tilletiopsis species, phyllosphere yeasts that are antagonists of powdery mildew fungi. Can J Microbiol. 2002;48:219-29. [ Links ]
44. Ye Y, Li Q, Fu G, Yuan G, Miao J, Lin W. Identification of antifungal substance (Iturin A2) produced by Bacillus subtilis B47 and its effect on southern corn leaf blight. J Integr Agric. 2012;11:90-9. [ Links ]














