Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista argentina de microbiología
Print version ISSN 0325-7541On-line version ISSN 1851-7617
Rev. argent. microbiol. vol.49 no.2 Ciudad Autónoma de Buenos Aires June 2017
http://dx.doi.org/10.1016/j.ram.2016.12.004
ORIGINAL ARTICLE
http://dx.doi.org/10.1016/j.ram.2016.12.004
Prevalence of honey bee (Apis mellifera) viruses in temperate and subtropical regions from Argentina
Prevalencia de virus en abejas melíferas (Apis mellifera) en ambientes templados y subtropicales de Argentina
Ana I. Molineri a, Adriana Pacini a, Agostina Giacobino a, Natalia Bulacio-Cagnolo b, Andrea Aignasse c, Luis Zago d, Norberto Fondevila e, Cecilia Ferrufino e, Julieta Merke b, Emanuel Orellano b, Ezequiel Bertozzi b, Hernán Pietronave b, Marcelo L. Signorini a,*
a. Consejo Nacional de Investigaciones Científicas y Técnicas, Instituto Nacional de Tecnología Agropecuaria EEA Rafaela, Rafaela, Santa Fe, Argentina
b. Instituto Nacional de Tecnología Agropecuaria EEA Rafaela, Rafaela, Santa Fe, Argentina
c. Ministerio de la Producción de la Provincia de Formosa, Programa para el Desarrollo Apícola, Formosa, Argentina
d. Instituto Nacional de Tecnología Agropecuaria EEA Resistencia, Resistencia, Chaco, Argentina
e. Instituto de Virología, Centro de Investigaciones en Ciencias Veterinarias y Agronómicas, Instituto Nacional de Tecnología Agropecuaria, Los Reseros y Las Cabañas, Castelar, Buenos Aires, Argentina
Received 12 July 2016; accepted 19 December 2016
Available online 25 April 2017
*Corresponding author.
E-mail address: marcelo.signorini@gmail.com (M.L. Signorini).
0325-7541/© 2017 Asociación Argentina de Microbiología. Published by Elsevier Espana, S.L.U. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Abstract
In Argentina, bee virus studies are still incipient, and there are no studies regarding the climatic effect. The aim of this study was to assess and compare the presence of honeybee viruses in different climatic regions from Argentina. A total of 385 colonies distributed in five Argentinean eco-regions were examined to evaluate the percentage of infestation with Varroa destructor and the presence of seven virus species (Deformed wing virus, DWV; Acute bee paralysis virus, ABPV; Chronic bee paralysis virus, CBPV; Black queen cell virus, BQCV; Kashmer bee virus, KBV; Israeli acute bee paralysis virus, IAPV; and Sacbrood bee virus, SBV) after honey yield. Two viruses, KBV and IAPV, were not detected. The other five viruses were found in different prevalences: DWV (35%), ABPV (21.5%), BQCV (8.0%), CBPV (2.2%), and SBV (1.1%). We found double and triple viral associations in approximately 25% of the sampled colonies. The mean V. destructor infestation in the colonies prior to the acaricide treatment was 7.12% ± 8.7%. The knowledge of the prevalence of these viruses in the region and their relation with the mite and other possible influencing factors is important for preventing colony losses. Further studies are necessary to identify the risk factors associated with virus presence and its relationship with other pathogens such as V. destructor.
Keywords
Deformed wing virus; Acute bee paralysis virus; Black queen cell virus; Varroa destructor; Environment.
Resumen
En Argentina, los estudios sobre prevalencia de virus en abejas continúan siendo incipientes y no existen reportes acerca de cómo inciden sobre dicha prevalencia las variables climáticas. El objetivo de este estudio fue evaluar y comparar la presencia de virus en abejas melíferas en diferentes regiones agroecológicas de Argentina. A tal fin se evaluaron 385 colmenas distribuidas en 5 regiones agroecológicas de las provincias de Chaco y Santa Fe; en ellas se analizó el porcentaje de infestación con Varroa destructor (ácaro patógeno de abejas) y la presencia de 7 especies de virus (DWV, virus de las alas deformadas; ABPV, virus de la parálisis aguda de la abeja; CBPV, virus de la parálisis crónica de la abeja; BQCV, virus de celda negra de la reina; KBV, virus de la abeja de Cachemira; IAPV, virus israelí de la parálisis aguda y SBV, virus de la cría ensacada). luego de la cosecha de miel. Dos virus (KBV y IAPV) no fueron detectados. Las otras 5 especies de virus se encontraron con prevalencias variables: DWV (35%), ABPV (21,5%), BQCV (8%), CBPV (2,2%) y SBV (1,1%). Fue posible identificar la presencia de 3 y hasta 3 virus simultáneamente en el 25% de las colmenas evaluadas. El promedio de infestación por V. destructor en las colmenas luego de la cosecha de miel y antes del tratamiento con acaricidas fue de 7,12% (±8,7). Conocer la prevalencia de virus en las diferentes regiones agroecológicas y su relación con la presencia del ácaro V. destructor e identificar otros posibles factores que podrían influir en su presencia es relevante para definir estrategias que reduzcan la mortandad de colmenas. Es necesario realizar estudios adicionales para identificar los factores de riesgo asociados a la presencia de virus en las colmenas y su relación con otros patógenos, como V. destructor.
Palabras clave
Virus de las alas deformes; Virus de la parálisis aguda; Virus de la celda real negra; Varroa destructor; Medioambiente.
Introduction
Several viruses are known as honey bee pathogens, most of which have been linked to Varroa destructor parasitism10,17,19,28. Generally, bee viruses do not produce any highly visible symptoms in the colony, causing covert infections10,19. Under stress conditions (unfavorable climate, pesticides, mismanagement or another pathogen such as V. destructor or Nosema sp. that could cause immunosuppression) they can produce overt infections, reducing lifespan of bees and causing visible symptoms10,19.
V. destructor is worldwide distributed and is considered the most relevant pathogen in Argentinean honey bee colonies, causing economic and productive damage20,36. Usually, V. destructor infestation is associated with virus infections17,18. Moreover, several honey bee viruses are transmitted by V. destructor mites including Deformed wing virus (DWV), Kashmir bee virus (KBV), Sacbrood bee virus (SBV), Acute bee paralysis virus (ABPV), and Israeli acute paralysis virus (IAPV)18.
In South America honeybee viruses were detected in different countries such as Uruguay2,3, Brazil38, and Chile5. In Argentina, honeybee virus studies are still incipient. To date, DWV, ABPV, SBV, IAPV, Black queen cell virus (BQCV), and Chronic bee paralysis virus (CBPV) have been detected in colonies located only in temperate climate9,21,31,32. Nevertheless, there are no studies about the prevalence of bee viruses in different climates that could be influencing their presence in the colonies. The aim of this study was to assess and compare the presence of honeybee viruses DWV, ABPV, KBV, CBPV, SBV, IAPV, and BQCV in colonies with different Varroa infestation levels in subtropical and temperate climate regions from Argentina.
Materials and methods
A cross-sectional study was carried out from February to June 2015 (autumn), in north-central Argentina. The sampling time was defined between the end of the honey production period and the beginning of the autumn acaricide treatment. At this moment, the colonies are commonly monitored14 because this is a key practice to guarantee healthy over-wintering conditions11. The study was carried out during an extended period (from February to June) because the honey harvest season and treatment time frame vary according to the geographical zone and the beekeeping management practices. A total of 385 colonies from 64 apiaries (owned by different beekeepers) were sampled. This number was consistent with the number of apiaries in the study area (n = 5300; 95% confidence level; precision = 10%, and 74% of expected prevalence of colonies with >3% of V. destructor infestation intensity during autumn)36. Apiaries were randomly chosen following stratified randomization procedures (computerized random numbers)28. Within each apiary, a minimum of six colonies or 10% of the total number of colonies (in apiaries larger than 60 colonies) were randomly selected to evaluate viruses and V. destructor infestation.
Five eco-regions were defined based on the nectar flow period and their beekeeping management schedule4,7,22,33,34. The eco-regions were defined as: South Santa Fe, Central Santa Fe, Humid Chaco, Transition Chaco, and Semi-arid Chaco (Table 1; Fig. 1). The number of colonies sampled in each eco-region was defined proportionally considering the total number of colonies in the region. Thus, the number of colonies sampled in each eco-region was: 48 in South Santa Fe, 102 in Central Santa Fe, 91 in Humid Chaco, 78 in Transition Chaco, and 66 in Semi-arid Chaco (Table 1).
Table 1. Region characterization based on annual mean temperature and precipitation, land use and floral resources

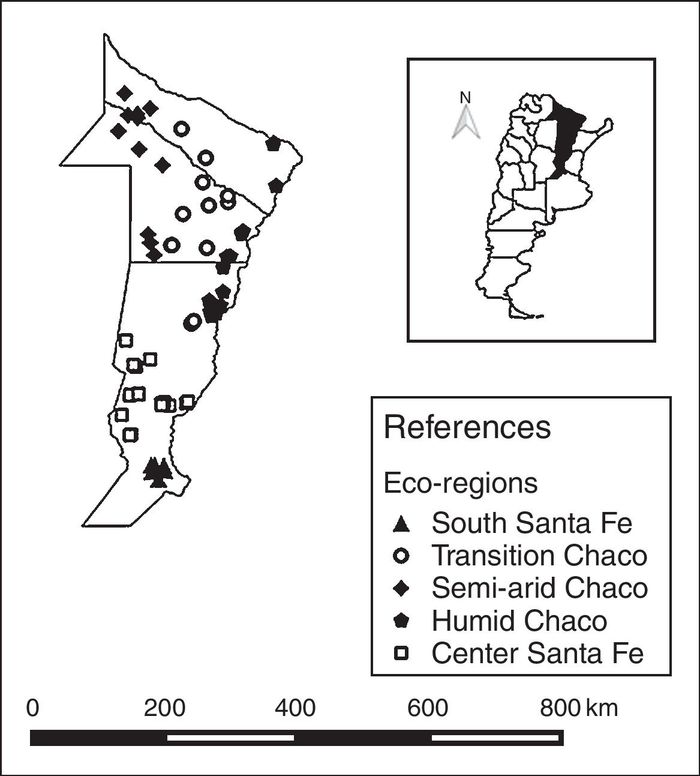
Figure 1. Location and distribution of apiaries according to eco-regions of Argentina.
Sampling and virus analysis
Approximately 40 nurse bees were collected from each colony and maintained alive in plastic containers with breathing holes until they were frozen at −20 °C. Live bees were used to ensure high-quality RNA1,18.
Pools of bees (n = 30) from each hive were macerated in mortar and homogenized with 7 ml of phosphate buffer (PBS) pH 7. The mixture was centrifuged at 4500 rpm at 8 °C for 45 min and the supernatant was collected and stored at −20 °C.
Viral RNA extraction
Total RNA was extracted using TRIzol Reagent, according to the recommendation of the manufacturer's protocol. RNA samples were dissolved in 10–50 µl ultra-pure water (DNAse, RNAse-Free Distilled Water; Invitrogen).
Reverse transcriptase reaction
Copy DNA was synthesized by reverse transcription reaction (RT) from the extracted RNA. The reaction mixture contained 1 µl of RNA (∼2 μg), 1 µl of reaction buffer 5× (Promega), 0.5 µl dNTP 10 mM (Promega), 0.125 µl of RNasin® 40 U/µl (Promega), 0.25 µl of random primers 2 μg/µl, 0.175 µl of reverse transcriptase 200 U/µl (Promega), and completed with a volume of 1.95 µl of ultra pure water (DNAse, RNAse-Free Distilled Water; Invitrogen) to obtain a total volume of 5 µl of mixture. The reaction was developed in a Biometra Trio-Thermoblock. The thermal cycling profiles were: 42 °C for 45 min, 94 °C for 10 min and 4 °C for 4 min.
qPCR amplification of DWV, ABPV, CBPV, BQCV, IAPV, SBV, and KBV virus
To determine the presence of DWV, BQCV, SBV, SBPV, CBPV, ABPV, KBV, and IAPV qPCR was carried out using the method described by Locke et al.26. Negative (H2O) and positive controls (recombinant plasmid DNA with the virus inserted into the pGEM-T Easy vector) were included in each run of the qPCR reaction. After amplification, a melting curve analysis was performed to determine the specificity of the PCR products. The housekeeping gene β-actin was used as an internal control, where the presence and quantification of this reference gene ensure that the entire procedure from extraction to quantification was performed without degradation of RNA18,26.
For qPCR amplification, the reaction mixture contained the primers described by Locke et al.27 (0.4 µl 1.5 uM of each pair of primers selected) master mix SYBR green PCR kit QuantiTect (cat 204143) 2.5 µl, 1.45 µl ultra-pure water (DNAse, RNAse-Free Distilled Water; Invitrogen), and 0.5 µl of cDNA. Samples were amplified using the LightCycler 2.0 Roche Thermocycler with the following thermal cycling profiles: 95 °C for 10 min, 45 cycles at 95 °C for 15 s and 56 °C for 1 min. The fluorescence emission of the samples was monitored at 530 nm. Samples having a geometric increase in fluorescence emission in the two previous successive cycles of cycling number 45 were considered positive. The first of this emission lifting cycle was considered as the first cycle of positivity (CP). Negative (H2O) and positive controls (recombinant plasmid DNA with the virus inserted into the pGEM-T Easy vector) were included in each run of the RT-PCR reaction.
The viral loads of positive samples were estimated using standard curves prepared with cycle threshold (Ct) data obtained from known concentrations of cDNA fragment copies of each virus studied. To convert the Ct values generated by qPCR from experimental samples to RNA genome copies per µl, serial 10-fold dilutions of in vitro RNA (synthesized using the primers described by Locke et al.27) from the plasmids (recombinant plasmid DNA with the virus inserted into the pGEM-T Easy vector) of known concentration were analyzed by the qPCR protocol described above. A linear relationship between the Ct crossing the threshold fluorescence and the log of the start molecules input in the reaction was done. The equation of the curve of RNA copy versus the normalized Ct value was used for subsequent conversions30.
Sampling and V. destructor analysis
Adult bees were examined to diagnose the presence of mites in all the colonies evaluated. In each colony, approximately 250 bees were collected from both sides of three unsealed brood combs in a jar containing 50% ethanol. The mites were separated from the bees by pouring the jar content into a sieve with a 2-mm mesh size15. The intensity of mite infestation on adult bees was calculated dividing the number of mites counted by the number of bees in the sample to determine the proportion of infested individuals and multiplying by 100 to obtain the percentage of infestation per colony15. In addition, the number of adult bees and number of cells with sealed brood, pollen, and honey of all colonies were estimated according to the Liebefeld method23.
Statistical analysis
In previous studies, we determined a critical threshold of 3% (mite load above the threshold which is recommended to treat colonies during autumn to avoid severe winter losses). Our results suggested that colonies that go through winter with more than 3% of mite load hardly survive until the following spring8,20. To establish a relative sanitary condition, previous results were used to subcategorize the colonies into two levels: high and low, according to their autumn infestation with mites (high: >3%; low: ≤3%).
A descriptive analysis was performed using the χ2 Test between the variable presence/absence of each virus and mite infestation compared between region. The same analysis was executed with V. destructor. Spearman correlation was performed between virus titers and mite infestation rate. Since it is not possible to log transform zero values, the response variable was Log10 of (virus copies + 1) in order to include all values (negative and positive samples). To determine the association between region (independent variable) and virus prevalence (dependent variable) a generalized linear mixed model (GLMM) with apiary as random effect (as all colonies from the same apiary are uniformly managed) was performed. Another GLMM was performed with apiary as random effect and each virus as dependent variable, but with V. destructor and region as independent variables. All statistical analyses were carried out using InfoStat software (Universidad Nacional de Córdoba, Argentina)25.
Results
The mean size of each apiary, bee population, frames cover with brood (FCBr), pollen (FCP) and honey (FCH) per region are available in Table 2.
Table 2. Descriptive data of the apiaries per region

a,b,c Different letters indicate significant difference (p < 0.05) for each eco-region.FCBr: frames covered with brood; FCP: frames covered with pollen; FCH: frames covered with honey.
The number of virus samples (n = 363) was lower than the estimated sample size (94.3%), which was due to bad climatic conditions hampering the access to the apiaries and to the fact that some samples were lost. Twenty-four of the samples of phoretic Varroa (PV) were missing (361 samples, 93.76%).
Neither KBV nor IAPV were detected in the analyzed colonies. The other five viruses were found in different prevalences: DWV (35%), ABPV (21.5%), BQCV (8.0%), CBPV (2.2%), and SBV (1.1%). Mean titers were 1.63 log10 virus/bee for DWV (SD = 2.02 log10 virus/bee), 0.42 log10 virus/bee for ABPV (SD = 1.17 log10 virus/bee), 0.37 log10 virus/bee for BQCV (SD = 6.98 log10 virus/bee), 0.023 log10 virus/bee for CBPV, and 0.002 log10 virus/bee for SBV (SD = 0.227 log10 virus/bee; SD = 0.044 log10 virus/bee).
In addition, we found an association between region and virus presence for ABPV (p < 0.001) and SBV (p = 0.040). Transition Chaco had higher mean titers of ABPV (0.83 log10 virus/bee) than the other regions and South Santa Fe had the lower ABPV mean titers (0.14 log10 virus/bee) (Fig. 2). South Santa Fe had SBV mean titers higher than the other regions (0.026 log10 virus/bee) (Fig. 2).

Figure 2. Deformed wing virus (DWV) and Acute bee paralysis virus (ABPV) relative virus level and mite infestation rate in honey bee colonies from Argentine Eco-regions (n =385).
Considering all the regions, the correlation between DWV and ABPV was the only significant among the viruses; however, the correlation coefficient was low (r = 0.369; p < 0.001).
Of the 363 colonies sampled, in 30.6% (n = 111) we did not detect any virus, 44.1% (n = 160) of them had only one virus, 22.3% (n = 81) had two viruses, and 3% (n = 11) had three viruses. The most common combination of two viruses were DWV with ABPV (n = 59) and DWV with BQCV (n = 13). Furthermore, the combination of DWV, ABPV, and BQCV was the most frequent triple virus co-infection (n = 9).
Two hundred and twenty out of the 361 colonies sampled for mite analysis (57.1%) showed an infestation with V. destructor higher than 3%. The mean infestation in the colonies prior to the acaricide treatment was 7.12% ± 8.7%. The region with the lowest mite infestation was Semi-arid Chaco (V. destructor media level = 3.01% ± 2.79, p = 0.008). Indeed, all Chaco regions (subtropical climate) had fewer colonies with >3% of V. destructor infestation than South and Central Santa Fe (temperate climate) (53.96% and 70.2%, respectively) (Fig. 2).
V. destructor infestation levels were correlated with DWV titers (r = 0.287, p < 0.001) and with ABPV titers (r = 0.112, p = 0.04). However, the correlation coefficients were low and the p-values may be influenced by the sample size.
An association between V. destructor infestation levels and DWV prevalence was found. When V. destructor levels were higher than >3%, DWV prevalence also increased (p = 0.019) (Table 3). A similar pattern was observed between ABPV and V. destructor infestation levels (p = 0.036) (Table 3). ABPV was also associated to the eco-region. Semi-arid Chaco had higher prevalence of virus than Humid Chaco and Central Santa Fe (p = 0.062; p = 0.027, respectively) (Table 3).
Table 3. Multivariable model using eco-regions, Varroa levels, ABPV, DWV and apiary data

The study included 385 hives in 64 apiaries form Argentina.a,b Different letters indicate significant difference (p < 0.05) for each eco-region.
Discussion
This is the first descriptive study concerning the distribution of honey bee viruses including apiaries from several eco-regions with subtropical and temperate climates. Moreover, it is the first study in Argentina which evaluated the relationship among different viruses and mites under five different agro-ecological conditions. Other studies were carried out in our country but were at small scale, all in temperate climate, and under a similar surrounding environment31,32. Another study conducted by our group21 described DWV presence in an apiary located in a subtropical zone but did not find other viruses. In this study, we included more apiaries located in two climates (temperate and sub-tropical) and under different surrounding environments. These differences may explain the identification of other viruses such as CBPV, ABPV, SBV, and BQCV, although in low prevalence. Previous studies also detected DWV, ABPV, CBPV, SBV, and BQCV in temperate climate from Argentina9,31,32. These studies have also detected IAPV, which was not detected in our study.
The most prevalent viruses detected in our study were DWV, ABPV, and BQCV. Similarly, DWV was the most prevalent virus in Uruguay, however it was found in 100% of the sampled colonies3. Alternatively, the same authors found that SBV was present in all the colonies whereas in this study SBV was the less prevalent virus. Our results are consistent with the observations reported by Weinstein Teixeira et al.37, in Brazil.
Multiple viral infections are frequently detected concomitantly in bee colonies6 and generally in an unapparent form of presentation24. We found double and triple viral associations in approximately 25% of the sampled colonies, which is a higher prevalence compared to previously reported results in our country29.
Honey bee viruses are extensively spread in the study area since almost 70% of the samples were positive to virus, they were detected in different eco-regions, and combined with several virus species. Generally, honeybee viruses can commonly be detected in healthy populations because they maintain themselves as covert infections13. Many of these viruses can multiply rapidly under stressful conditions and cause a disease. This situation usually arises when the colony is threatened by external stressors such as infestation with V. destructor35. Additional studies should be conducted with the aim to identify the most important factors associated with the prevalence of the viruses in the different regions.
All regions showed similar prevalence of DWV but different ABPV prevalence and V. destructor infestation level. The mite infestation level found prior to treatment was the expected one according to the apiculture productive cycle. The lower mite infestation observed in subtropical climate may be supported by a higher impact of the Africanized bees in subtropical colonies33. A higher level of hygienic behavior, lower levels of mite reproduction on pupae, and higher levels of grooming mites off adult bees than European bees have been observed in Africanized bees from South America23.
It is well known that DWV could appear in regions were V. destructor has not been reported39. Moreover, De Miranda and Genersch13 stated that the presence of DWV with no visible symptoms may be observed independent of V. destructor. Furthermore, in our study V. destructor–virus correlations were significant but they were very low. Colonies having more than 3% of V. destructor had more virus prevalences; however, this is not a linear relationship. This might be explained by the fact that, even when V. destructor is indeed a possible vector for ABPV and DWV19, these viruses replicate and transmit using other mechanisms13,27,37. Other study16 found higher correlation levels between V. destructor and DWV. Meixner et al.28, found that V. destructor infestation level in autumn did not contribute to the presence of DWV and ABPV. They observed an association between V. destructor infestation level and the presence of viruses; however the presence of many other factors influencing this relationship (management practices, climate or environmental conditions) was evident. For instance, in our study ABPV was more prevalent in Semi-arid Chaco where V. destructor infestation level was low. However, this is a region with high average annual temperatures and a long and active foraging season. Similar results were found in other studies, where ABPV was more prevalent under similar environmental conditions28.
Another possible explanation may be related to the nutritional condition in the hives located in subtropical climate. Indeed, Transition and Semi-arid Chaco had more pollen and honey reserves than the other eco-regions. Nutritional status has been identified as a factor which impacts on colony health12,20.
Virus prevalence is multifactorial, being influenced by several factors, including climatic and environmental conditions, concomitant infections (V. destructor, Nosema sp.) and their interactions. Further studies are needed to identify the risk factors associated with virus presence and its relationship with other pathogens.
Ethical responsibilities
Protection of human and animal subjects
The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data
The authors declare that no patient data appear in this article.
Right to privacy and informed consent
The authors declare that no patient data appear in this article.
Conflict of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
This study has been carried out with the financial support of: a) PNAPI Project No. 1112042 and Specific Project No. 1112042 "Estrategias multidisciplinarias para mitigar el efecto del nuevo contexto ambiental y productivo sobre la colmena", Instituto Nacional de Tecnología Agropecuaria; b) Premio SENASA "Risk factors associated with the presence, distribution and interaction of the main diseases affecting beekeeping in sub-tropical and temperate environments". We would like to thanks to B. Locke (Swedish University of Agricultural Sciences) and W. Shi (Bee Research Institute, CAAS) for giving us the plasmids used as control for virus analysis. Ana Molineri, Agostina Giacobino, and Adriana Pacini are doctoral fellows from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina). Dr. Marcelo L. Signorini is a Research Career Member from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina).
1. Amiri E, Meixner M, Nielsen SL, Kryger P. Four categories of viral infection describe the health status of Honey bee colonies. PLOS ONE. 2015;10:e0140272, http://dx.doi.org/10.1371/journal.pone.0140272
2. Antúnez K, D' Alessandro B, Corbella E, Zunino P. Detection of chronic bee paralysis virus and acute bee paralysis virus in Uruguayan honeybees. J Inv Pat. 2005;90:69-72. [ Links ]
3. Antúnez K, D' Alessandro B, Corbella E, Ramallo G, Zunino P. Honeybee viruses in Uruguay. J Inv Pat. 2006;93:67-70. [ Links ]
4. Arzamendia V, Giraudo AR. Usando patrones de biodiversidad para la evaluación y diseno de áreas protegidas: las serpientes de la Provincia de Santa Fe (Argentina) como ejemplo. Rev Chil Hist Nat. 2004;77:335-48. [ Links ]
5. Barriga GP, Cifuentes-Munoz N, Rivera PA, Gutiérrez M, Shmaryahu A, Valenzuela PDT, Engel EA. First detection and complete genome sequence of deformed wing virus in Chilean honeybees. Virus Genes. 2012;45:606-9, http://dx.doi.org/10.1007/s11262-012-0791-0 [ Links ]
6. Berényi O, Bakonyi T, Derakhshifar I, Köglberger H, Nowotny N. Occurrence of six honeybee viruses in diseased Austrian apiaries. Appl Environ Microbiol. 2006;72:2414-20. [ Links ]
7. Burkart R, Bárbaro NO, Sánchez RO, Gómez DA. Ecoregiones de la Argentina. Administración de Parques Nacionales. Buenos Aires. 1999, http://www.sib.gov.ar/archivos/Eco-Regiones_de_la_Argentina.pdf [accessed 10.12.12].
8. Bulacio Cagnolo NV [PhD thesis] Manejo Integrado de Varroa destructor (Acari: Varroidae) en colonias de Apis mellifera (Hymenoptera: Apidae) en el centro oeste de la provincia de Santa Fe. Argentina: Facultad de Ciencias Naturales, Universidad Nacional de Mar del Plata; 2011.
9. Castilla R, Reynaldi FJ, Sguazza GH, Pecoraro MR, Galosi CM. Detección de virus que afectan a las abejas durante el periodo 2009-2014. In: XI Congreso Argentino de Virología and II Congreso Latinoamericano de Virología. 2015. [ Links ]
10. Chen YP, Siede R. Honey bee viruses. Adv Virus Res. 2007;70:33-80. [ Links ]
11. Currie RW, Gatien P. Timing acaricide treatments to prevent Varroa destructor (Acari: Varroidae) from causing economic damage to honey bee colonies. Can Entomol. 2006;138:238-52. [ Links ]
12. DeGrandi-Hoffman G, Chen Y, Rivera R, Carroll M, Chambers M, Hidalgo G, Watkins de Jong E. Honey bee colonies provided with natural forage have lower pathogen loads and higher overwinter survival than those fed protein supplements. Apidologie. 2016;47:186-96. [ Links ]
13. De Miranda JR, Genersch E. Deformed wing virus. J Inv Pat. 2010;103:S48-61. [ Links ]
14. Department of Agriculture from Santa Fe province. Cadena apícola santafecina; 2008. http://www.santafe.gov.ar/index.php/web/content/download/66066/320676/file/descargar.pdf [in Spanish; accessed 17.05.16].
15. Dietemann V, Nazzi F, Martin SJ, Anderson DL, Locke B, Delaplane KS, Wauquiez Q, Tannahill C, Frey E, Ziegelmann B, Rosenkranz vP., Ellis JD. Standard methods for Varroa research. In: Dietemann V, Ellis JD, Neumann P, editors. The COLOSS BEEBOOK, Vol. II: standard methods for Apis mellifera pest and pathogen research. 2013. J. Apic. Res. 52, http://dx.doi.org/10.3896/IBRA.1.52.1.09.
16. Di Prisco G, Zhang X, Pennacchio F, Caprio E, Li J, Evans JD, Degrandi-Hoffman G, Hamilton M, Chen YP. Dynamics of persistent and acute deformed wing virus infections in honey bees, Apis mellifera. Viruses. 2011;3:2425-41, http://dx.doi.org/10.3390/v3122425 [ Links ]
17. Emsen B, Hamiduzzaman MM, Goodwin PH, Guzman-Novoa E. Lower virus infections in Varroa destructor-infested and uninfested brood and adult honey bees (Apis mellifera) of a low mite population growth colony compared to a high mite population growth colony. PLOS ONE. 2015;10:e0118885, http://dx.doi.org/10.1371/journal.pone.0118885
18. Francis RM, Nielsen SL, Kryger P. Varroa-virus interaction in collapsing honey bee colonies. PLoS One. 2013;8:e57540, http://dx.doi.org/10.1371/journal.pone.0057540 PMID:23526946.
19. Genersch E, Aubert M. Emerging and re-emerging viruses of the honey bee (Apis mellifera L.). Vet Res. 2010;41:54. [ Links ]
20. Giacobino A, Bulacio Cagnolo N, Merke J, Orellano E, Bertozzi E, Masciangelo G, Pietronave H, Salto C, Signorini M. Risk factors associated with the presence of Varroa destructor in honey bee colonies from east-central Argentina. Prev Vet Med. 2014;115:280-7. [ Links ]
21. Giacobino A, Molineri A, Pacini A, Fondevila N, Pietronave H, Rodríguez G, Palacio A, Bulacio Cagnolo N, Orellano E, Salto CE, Signorini ML, Merke J. Varroa destructor and viruses association in honey bee colonies under different climatic conditions. Environ Microbiol. 2016;8:407-12, http://dx.doi.org/10.1111/1462-2920.13297 [ Links ]
22. Giorgi R, Tosolini R, Sapino V, Villar J, León C, Chiavassa A. Zonificación Agroeconómica de la provincia de Santa Fe, vol.110. Argentina: INTA; 2008. p. 215-24. ISSN 0325-9137. [ Links ]
23. Guzman-Novoa E, Vandame R, Arechavaleta ME. Susceptibility of European and Africanized honey bees (Apis mellifera L.) to Varroa jacobsoni Oud. In Mexico. Apidologie. 1999;30:173-82. [ Links ]
24. Hung ACF, Shimanuki H, Knox DA. Inapparent infection of acute paralysis virus and Kashmir bee virus in the US honey bees. Am Bee J. 1996;136:874-6. [ Links ]
25. Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW. INFOSTAT versión 2011. Argentina: Grupo InfoStat, FCA, Universidad Nacional de Córdoba; 2011. URL http://wwwinfostat.com.ar. [ Links ]
26. Locke B, Forsgren E, Fries I, de Miranda JR. Acaricide treatment affects viral dynamics in Varroa destructor-infested honey bee colonies via both host physiology and mite control. Appl Environ Microbiol. 2012;78:227-35. [ Links ]
27. Locke B, Le Conte Y, Crauser D, Fries I. Host adaptations reduce the reproductive success of Varroa destructor in two distinct European honey bee populations. Ecol Evol. 2012;2:1144-50. [ Links ]
28. Meixner MD, Francis RM, Gajda A, Kryger P, Andonov S, Uzunov A, Topolska G, Costa C, Amiri E, Berg S, Bienkowska M, Bouga M, Büchler R, Dyrba W, Gurgulova K, Hatjina F, Ivanova E, Janes M, Kezic N, Korpela S, Le Conte Y, Panasiuk B, Pechhacker H, Tsoktouridis G, Vaccari G, Wilde J. Occurrence of parasites and pathogens in honeybee colonies used in a European genotype-environment interactions experiment. J Apic Res. 2014;53:215-29, http://dx.doi.org/10.3896/IBRA.1.53.2.04 [ Links ]
29. Moher D, Hopewell S, Schulz K, Montori V, Gotzsche P, Devereaux PJ, Elbourne D, Egger M, Altman DG. ConSoRT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010, http://dx.doi.org/10.1136/bmj.c869 [ Links ]
30. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11328886. [ Links ]
31. Reynaldi FJ, Sguazza GH, Pecoraro MR, Tizzano MA, Galosi CM. First report of viral infections that affect argentine honeybees. Environ Microbiol Rep. 2010;2:749-51. [ Links ]
32. Reynaldi FJ, Sguazza GH, Tizzano MA, Fuentealba N, Galosi CM, Pecoraro MR. First report of Israeli acute paralysis virus in asymptomatic hives of Argentina. Rev Argent Microbiol. 2011;43:84-6. [ Links ]
33. RIAN (Red de Información Agropecuaria Nacional). Zonificación RIAN Chaco y Formosa. Publicación INTA; 2010. [ Links ]
34. Riveros F; 2009. El gran Chaco. http://www.fao.org/ag/agp/agpc/doc/counprof/spanishtrad/argentina_sp/granchaco/GranChaco_sp.htm [accessed December 2015].
35. Rosenkranz P. Honey bee (Apis mellifera L.) tolerance to Varroa jacobsoni Oud. In South America. Apidologie. 1999;30:159-72. [ Links ]
36. SENASA. Situación actual de Varroosis; 2007. http://www.senasa.gov.ar/Archivos/File/File3824-varroosis-aituacionactual-argentina.pdf [accessed March 2011].
37. Sumpter DJT, Martin SJ. The dynamics of virus epidemics inVarroa-infested honey bee colonies. J An Ecol. 2004;73:51-63. [ Links ]
38. Weinstein Teixeira E, Chen Y, Message D, Pettis J, Evans JD. Virus infections in Brazilian honey bees. J Inv Pat. 2008;99: 117-9. [ Links ]
39. Yue C, Genersch E. RT-PCR analysis of Deformed wing virus in honeybees (Apis mellifera) and mites (Varroa destructor). J Gen Virol. 2005;86:3419-24. [ Links ]














