Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista argentina de microbiología
Print version ISSN 0325-7541On-line version ISSN 1851-7617
Rev. argent. microbiol. vol.49 no.3 Ciudad Autónoma de Buenos Aires Sept. 2017
http://dx.doi.org/10.1016/j.ram.2016.12.009
ORIGINAL ARTICLE
http://dx.doi.org/10.1016/j.ram.2016.12.009
Fungal endophytes isolated from Protium heptaphyllum and Trattinnickia rhoifolia as antagonists of Fusarium oxysporum
Endófitos fúngicos aislados de Protium heptaphyllum y Trattinnickia rhoifolia como antagonistas de Fusarium oxysporum
Juan E. Fierro-Cruza, Pedro Jiméneza, Ericsson Coy-Barreraa,*
a Universidad Militar Nueva Granada, Km 2 Cajica - Zipaquira, Cajica, Colombia
Received 8 February 2016; accepted 28 December 2016
Available online 8 May 2017
*Corresponding author.
E-mail address: inquibio@unimilitar.edu.co (E. Coy-Barrera).
0325-7541/© 2017 Asociacion Argentina de Microbiología. Published by Elsevier España, S.L.U. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
ABSTRACT
Control of fungal pathogens is mainly addressed by the use of chemically synthesized fungicides which result in environmental pollution, developing resistance after prolonged use. In this context, endophytes have been recognized as potential biocontrollers, and also as a promising source of antifungal metabolites. Therefore, as part of our research on phytopathogen controllers, 355 fungal endophytes were isolated from Protium heptaphyllum and Trattinnickia rhoifolia (Burseraceae), both ethnobotanically important tree species that produce secondary metabolites of agronomic and industrial interest. Endophytes were tested by in vitro dual culture against Fusarium oxysporum, a phytopathogen of agronomic importance. Five endophytes exerted at least 40% inhibition on F. oxysporum growth. Ethyl acetate (EtOAc) extracts were obtained from the most active antagonistic fungi, after growing them in three different liquid media. The extracts were tested against a conidial suspension of F. oxysporum by direct bioautography. Two extracts derived from fungi identified as Chaetomium globosum, F211JJMNG and Meyerozima sp. F281.UMNG showed inhibition of pathogen growth. Isolate C. globosum, F211JJMNG was selected for a chemical analysis by RP-HPLC-DAD-ESI-MS and antifungal molecules such as cladosporin, chaetoatrosin A and chaetoviridin A were annotated and identified based on their MS data.
KEYWORDS
Endophytes; Burseraceae; Chaetomium; Metabolites
RESUMEN
El control de patógenos fúngicos se basa principalmente en el uso de fungicidas de síntesis química, los que pueden dar lugar a la contaminación del medio ambiente y el desarrollo de resistencia después de un uso prolongado. En este contexto, los endófitos han sido reconocidos como potenciales biocontroladores y también como fuentes prometedoras de metabolitos secundarios antifúngicos. En el marco de nuestra investigación sobre controladores de fitopatógenos, se aislaron 355 hongos endófitos de Protium heptaphyllum y Trattinnickia rhoifolia (Burseraceae), especies arbóreas de valor etnobotánico que producen metabolitos secundarios de interés agronómico e industrial. Los endófitos fueron evaluados in vitro en cultivos duales frente a Fusarium oxysporum, un fitopatógeno de importancia agronómica. Cinco endófitos mostraron al menos un 40% de inhibición en el crecimiento de F. oxysporum. Una vez determinados los hongos más activos, estos se cultivaron en 3 medios líquidos diferentes y a partir de ellos se preparó una serie de extractos solubles en acetato de etilo. Los extractos fueron probados contra una suspensión de conidios de F. oxysporum por bioautografía directa. Dos extractos derivados de los hongos identificados como Chaetomium globosum (F211.UMNG) y Meyerozima sp. (F281JJMNG) mostraron inhibición del crecimiento del patógeno. En el extracto derivado del hongo C. globosum se anotaron e identificaron los compuestos antifúngicos cladosporina, chaetoatrosina A y chaetoviridina A mediante el análisis por RP-HPLC-DAD-ESI-MS.
PALABRAS CLAVE
Endófitos; Burseraceae; Chaetomium; Metabolitos
Introduction
Using chemically synthesized fungicides has been the first line strategy to control phytopathogenic fungi31. However, secondary effects, such as environmental pollution and resistance development due to the use of these products, has led to a growing reluctance to use hazardous fungicides in agriculture. Thus, an enhanced trend in searching new control strategies involving environment-friendly alternatives in the management of plant pathogens has arisen20. In the search for such control strategies, naturally-occurring chemical entities have become potential alternatives for the industry to replace synthetic products23. In this context, microorganisms constitute a rich source of compounds with useful properties48 for several applications in the agrochemical and pharmaceutical industries11,23.
For several decades, the interaction between fungal endophytes and their hosts has attracted the researchers' attention, mainly because of the advantageous characteristics they confer to their host. Among these characteristics we can mention enhanced stress tolerance, plant growth factor production, herbivore repellency and protection against pathogens18. The latter characteristic is partly due to the fact that endophytes compete with other microorganisms for a specific niche, which could be achieved by the production of antibiotic-like secondary metabolites, along with other strategies4. As a consequence of their repellent properties, endophytes have been proposed as biocontrollers and as a promising source of antifungal metabolites against phytopathogens of agronomic importance18.
Based on our ongoing search for biologically active secondary metabolites from endophytic fungi, the objective of this work was to explore the diversity of endophytes isolated from Protium heptaphyllum and Trattinnickia rhoifolia (Burseraceae) form Casanare, Colombia. These tree species, have been traditionally used by indigenous communities to treat several ailments12, and their complex chemical repertory has provided useful compounds having industrial, pharmaceutical and agronomic potential38,43. Furthermore, endophytes have been isolated from a species of the Burseraceae family, such as Muscodor yucatensis25, with potential for controlling phytopathogens. Therefore, the aim of this work was to test in vitro the abilities of endophytes to inhibit the mycelial growth of Fusarium oxysporum, by metabolite production. F. oxysporum is a pathogen of many plant species that represent a major threat for the production of several agriculturally important crops, such as banana, carnation, chickpeas, dates, lentils, tomato, and others27. The active component or components, responsible for the antifungal activity were partially characterized following a bioassay-guided fractionation test of the liquid culture-derived crude extract from the most antagonistic endophyte, to be incorporated in the future to control management programs for plant pathogen F. oxysporum.
Methods
Recovery of endophytes and isolation
A total of two individuals from P. heptaphyllum and two from T. rhoifolia were collected in the foothill of the west Colombian Andes mountains in Aguazul, Casanare, Colombia (N 05°13"47.89", W 072°30'31.38"), a transition ecosystem between the savanna and the high Andean ecosystems. Botanical specimens of P. heptaphyllum (Aubl.) Marchand (COL573961) and T. rhoifolia (Aubl.) Marchand (COL573962) were deposited in the Colombian National Herbarium. From each tree, the plant material (from higher, medium and lower strata) was sampled in order to collect representative isolates from all the plants. Five leaves per level were collected in a total of 60 leaflets that were bagged in sealed bags and stored in dark conditions for 24 h at room temperature (ca. 26°C). Petioles were then removed and the complete leaves were vigorously washed with distilled sterile water and Tween 20 (0.01%), then submerged in 70% aqueous ethanol (1 min), then in 1% sodium hypochlorite (3 min), and then rinsed three times with sterilized distilled water. Leaves were then imprinted on Potato Dextrose Agar (PDA, Oxoid, UK) for verifying the disinfection of all epiphytic microorganisms.
Each leaf was sectioned into 2 mm2 pieces, and 5 randomly-chosen pieces from each leaf were seeded in Petri dishes (90 mm x 15 mm) containing water agar (agar 1.5%) (WA), 1/10 PDA (PDA at a 10th of the recommended concentration) or PDA, and then incubated at 26°C. Hyphae tips emerging from the leaf pieces were collected for three weeks, and sub-cultured on PDA at 26°C in the dark. Axenic cultures were established and, when a specific fungus sporu-lated, a monosporic culture was established. Those fungi that never sporulated were kept for a hyphal tip culture.
Identification of endophytes
Fungal populations were identified on the basis of cultural characteristics and morphology of fruiting bodies and spores3,14,21. Fungi were identified up to the genus level by observing the presence of conidial mycelium, spore mass color, distinctive reverse colony color, production of diffusible pigments, and spore morphology3. Cultures that repetitively failed to sporulate on different media were recorded as mycelia sterilia.
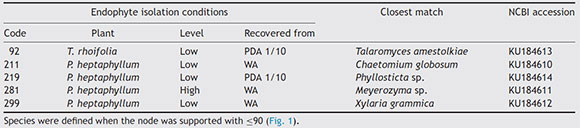
Additionally, those endophytes that inhibited F. oxysporum growth above 40% were identified by amplification of the nuclear ribosomal internal transcribed spacer (ITS) region, using the primers ITS1 (5 -TCC GTA GGT GAA CCT GCG G-3 ) and ITS 4(5 -TCC TCC GCT TAT TGA TAT GC-3 )46. Amplicons were sequenced with the same primers bidirectionally a single time in Macrogen Inc. (Korea), and the resulting sequences were aligned and edited in BioEdit v7.2.513. The sequences were confronted with those in GeneBank database (http://www.ncbi.nlm.nih.gov), using BLASTN 2.2.2828. The closest match was selected and aligned using ClustalW26. For the phylogenetic analysis, tree constructions were done with the MEGA 6.0 program package41 using the neighbor-joining method37. Bootstrap analysis was done using 1000-times resampled data. The resulting sequences were deposited in the GenBank.
Ethyl acetate (EtOAc) extraction
Fungi selected for their inhibitory activity at in vitro conditions against F. oxysporum were reactivated in 500 ml of Potato Dextrose Broth (PDB, Oxoid, UK), Sabouraud Broth (SAB, Oxoid, UK) and yeast extract sucrose media (YES)19, and cultured in an orbital shaker under constant agitation (100 rpm) at 21°C for 7 days. After that period the culture was filtered using Whatman No. 1 qualitative filter paper, and mycelia were lyophilized. Separately, both mycelia and filtered media were mixed with EtOAc in a 1:3 proportion and incubated in an orbital shaker in constant agitation (100 rpm) for 48 h. The organic phase (EtOAc) was separated from the mycelia by vacuum filtration using Whatman No. 1 qualitative filter paper, and from filtered liquid media using a decantation funnel. The resulting extracts were concentrated by lyophilization.
Antifungal assays
Fungal endophytes and a phytopathogenic isolate (F oxysporum G1 isolated from Physalis peruviana (Cape gooseberry) available in the collection of the phytopathology laboratory at Universidad Militar Nueva Granada) were cultured on PDA at 26°C for 5 days at 26°C in the dark. In order to evaluate the possible effect of each endophyte on phytopathogen growth, dual cultures were settled and each isolate was challenged with F. oxysporum G1. Thus, a plug (3 mm diameter), which was obtained from the colonial actively growing edge of the endophyte to be tested, was seeded on PDA, 10 mm away from the edge of a Petri dish (90mm x10mm). At a spot distance (10mm) from the diametrally-opposed edge, a similar plug of F. oxysporum was seeded. Six days later, the effect of each endophyte on F. oxysporum growth was observed and F. oxysporum colony radial measurement and distance between colonies were recorded. As control, a plug of each organism was cultured alone. These experiments were replicated three times. Results were compared by the Tukey's HSD (honest significant difference) test.
The antifungal activity of the extracts was tested by direct TLC bioautographic detection9. Extracts and fractions from the selected endophytes (Table 1) were diluted in ethanol (HPLC grade) and 30 µg were seeded in a single spot on a TLC Aluminum silica gel 60 Sheet 20 cm x 20cm (Sigma-Aldrich). Then the silica sheet was sprayed with a 1 x 106conidia/ml conidial suspension of F. oxysporum until the whole sheet was covered. The assays were incubated in a humid chamber for 3 days, and then F. oxysporum growth over the sheet was evaluated under UV-light.
Table 1 Isolation media and closest match in phylogenetic analysis by the neighbor joining method form the most active endophytes against F. oxysporum
Fractionation of the most active extract
The most active extract was fractionated by preparative HPLC (Shimadzu prominence LC20AD), in gradient elution, using a Shimadzu Premier column C-18 (4.6 mm x 150 mm, 5 µm) at a flow rate of 2 ml/min. The injection volume was 50 p,l. The mobile phases consisted in methanol (HPLC grade) (Phase A) and trifluoroacetic acid 0.005% (HPLC grade) (Phase B). Separation was carried out for 25 min, in a FRC10A Shimadzu fraction collector. A diode array detector (DAD) performed signal detection at 270 nm. A total of 20 fractions were recovered and then concentrated by lyophilization.
LC-MS-based chemical analysis
Extracts and fractions were characterized by Reverse Phase Liquid Chromatography with multi-wavelength UV-VIS detection (by a DAD) and coupled by electrospray to a mass spectrometry detector (RP-HPLC-DAD-ESI-MS) (Shimadzu Prominence LC/MS 8030). Analyses were performed on a Shi-madzu prominence instrument, in gradient elution, using a Shimadzu Premier column C-18 (4.6mm x 150mm, 5µm). Simultaneous monitoring was carried out at 270 nm, at a flow rate of 0.6ml/min. The operating temperature was 30°C and the injection volume was 20 µl. As mobile phase A 1% formic acid in distilled water (HPLC grade) was used, and acetonitrile (ACN) (HPLC grade) as mobile phase B; separation was performed for 33 min. The mass spectrometry detector (MSD) consisted of an electrospray ionization (ESI) source and a triple quadrupole analyzer. The mass spectrometry method consisted of a scan in simultaneous positive and negative ionization with an acquisition time of 2-33 min, a mass range of 50-2000 m/z, a scan speed of 1667 ^/s, an event time of 0.5 s, nebulizer gas flow of 1.5l/min, 350°C interface temperature and DL, and 450°C block temperature. The drying gas flow rate was 9 l/s. The analysis was monitored at wavelengths between 270 and 330nm. Annotation and identification of the major and minor metabolites in the extract was performed by mass spectrometry- based analysis, complemented with the analysis of reported metabolites.
Results
Recovery of endophytes
A total of 577 endophytes were isolated from 900 cultured pieces of leaflets. A subtotal of 355 endophytes were selected after the elimination of redundant morphotypes derived from the same leaflet. The highest number of endophytes (n = 236) was recovered from the lowest collection level for both species. The ITS region of the isolates was amplified and sequenced to determine the phylogenetic relationships among them (Fig. 1). A phylogenetic tree was constructed based on a 570 bp sequences and isolates clustered as follows: endophyte F92.UMNG clustered with Talaromyces amestolkiae, F18_UMNG with Phyllosticta sp., F211_UMNG with Chaetomium globosum, F299_UMNG with Xylaria grammica, and F281.UMNG with Meyerozyma sp. (Table 1).
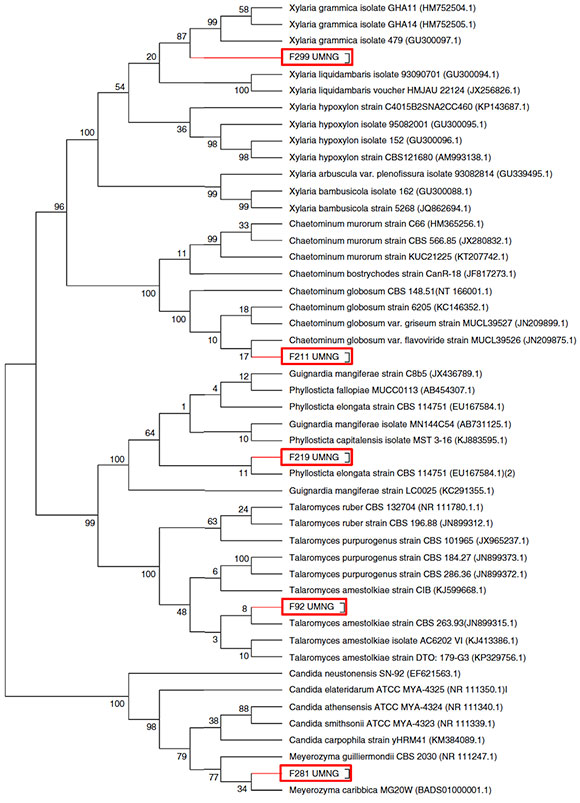
Figure 1 Dendrogram showing the phylogenetic relationship of fungal endophytes based on the ITS region. Phylogenies were inferred using the neighbor-joining analysis and trees generated in MEGA 6.0 software. Numbers at branch points indicate bootstrap values. The scale bars represent the estimated difference in nucleotide sequence. Red rectangles indicate the endophytes isolated in this work.
Antifungal test
The antifungal ability of 355 fungal endophytes against F. oxysporum G1 was evaluated by the dual culture method. Five endophytes reduced the area of F. oxysporum growth by at least 40%, without colony contact (Fig. 2), which were grouped in a single group by the Tukey's HSD test.
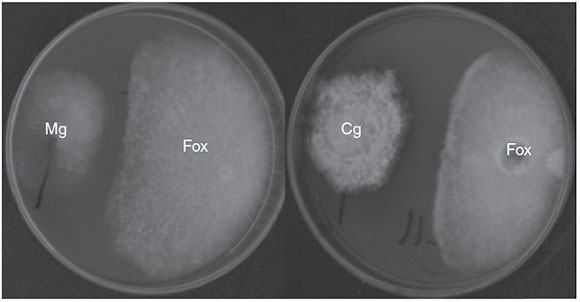
Figure 2 Inhibition of F. oxysporum G1 (Fox) caused by C. globosum F211 UMNG (Cg) and Meyerozima sp. (Mg) in PDA media indual cultures at 6 days post inoculation.
EtOAc-soluble extracts obtained from two isolates, F211_UMNG and F281_UMNG, cultured in YES, exerted inhibitory effect on F. oxysporum by direct bioautography (Fig. 3A). On comparing the inhibitory effect produced by these two isolates on F. oxysporum radial growth, it was found that isolate F211.UMNG (C. globosum) exerted a 64% in vitro inhibition of F. oxysporum colony growth arresting its growth producing with a distance between colonies of 12.5 ±0.6 mm while F281 _UMNG caused 45% inhibition showing 5.7 ±0.9 mm between colonies (Figs. 2 and 3A).
Figure 3 (A) Direct bioautography of endophyte-derivedEtOAc extracts against F. oxysporum conidia. Solid lines: Procloraz 40 ng (control). Medium dashed line: Supernatantmedium of EtOAc extract from C. globosum F211 UMNG.Highly dashed line: Supernatant medium of EtOAc extract from M. guilliermondii F281UMNG. (B) Bioautography of F211 UMNG extract and most active fraction against F. oxysporum conidia.A: Fraction #14, B: Extract 211 INI, C: Sportak 40 ng.
Since the extract from F211 _UMNG isolate showed greater inhibitory activity against F. oxysporum, it was fractionated in order to determine the most active fractions. A total of 20 fractions were recovered (Fig. 4A) and were additionally tested by bioautography against a conidial suspension of F. oxysporum. Only fraction #14 (30 µg) exerted inhibition of the fungus (Fig. 3B). Fraction #14 was analyzed by LC-MS, rendering a chromatogram that included two defined signals between min 12 and min 17 (peaks 1 and 3, Fig. 4B).

Figure 4 (A) Chromatographic profile of EtOAc extract from F211 UMNG in YES medium. F14+ arrow indicates fraction #14. (B)RP-HPLC-DAD chromatogram of fraction #14. Numbers indicate the annotated compounds by MS data (Table 2). (C) Structures ofthe identified compounds in the most active fraction from EtOAc-soluble extract of C. globosum F 211 UMNG.
Discussion
From the recovered isolates, fungi like Alternaría sp., Aspergillus sp., Chaetomium sp., Epicoccum sp., Fusarium sp., Pestalotiopsis sp., Phomopsis sp., Xylaria sp., among others, were identified by their morphological traits and have been previously reported as common endophytes in other plants6"8,22,24,29,47,49. A high diversity of fungal species were also found in leaves and stems of Boswellia sacra (Burseraceae), being Alternaria and Aspergillus the most dominant genera, which were both also isolated in this work. However, Chaetomium was also found in a relative high proportion (26.3%) represented by two species, C. globosum and C. spirale10. Screening works in Boswellia serrata exhibited Myrothecium verrucaria and Phoma sp.40 as dominant endophytes, which were also isolated in our samples.
The 355 endophytes isolated in this work were evaluated by the dual culture method against F. oxysporum and only five endophytes showed inhibition against F. oxysporum presumably by metabolite production because they inhibited the extension on the colony without mycelial contact and reduced the area of the phytopathogen by at least 40% (Table 1, Fig. 2). Antagonistic endophytes were identified by amplification of a 570 bp (ITS) region as C. globosum, Meyerozyma spp., Phyllosticta spp., T. amestolkiae, and X. grammic; such species have been reported as being endophytes and having antibiotic activity1,2,17,33,35,39,49.
The extract from isolate C. globosum F211.UMNG, at 30 µg, caused inhibit they inhibited the extension on the colony ion of F oxysporum growth (Fig. 3A). Based on the available literature, in the Chaetomium genus, mostly in C. globosum, seven signals defined by mass spectrum analysis were found to have the same m/z value to that reported (Table 2). Nevertheless, five isomers previously reported in the Chaetomium genus matched the m/z value detected at 38 min (peak 5, Fig. 4B) and, therefore they cannot be differentiated in accordance with the known MS limitations. Previous studies found that C. globosum synthetized several molecules such as chaetoglobosins, epipolythiodi-oxopiperazines, azaphilones, xanthones, anthraquinones, chromones, depsidones, terpenoids, and steroids, among others. These types of compounds have shown antitumor, cytotoxic, antimalarial, enzyme inhibitory, antibiotic, and other activities50. In the present study cladosporin, chaetoa-trosin A and chaetoviridin A (Fig. 4C) were identified to be active against F. oxysporum, in the EtOAc extract of C. globosum F211_UMNG. These compounds were previously reported as having antifungal activity16,34,45. Chaetoatrosin A acts as an inhibitor of chitin synthase II, while chaetoviridin A inhibits the cholesteryl ester transfer protein (CETP)42. The action of cladosporin is no fully understood but it has been reported that it exhibited lysyl-tRNA synthetase inhibition in P. falciparum15 and that its mode of action is different to that affecting^-tubuline assembly in mitosis45. The combination of the modes of action of the identified molecules might rationalize the observed growth inhibition of F. oxysporum in the in vitro and bioautography test.
Table 2 Identified compounds isolated from other Chaetomium strains as constituents of fraction #14

In conclusion, five endophytes acting as antagonists of F. oxysporum under in vitro conditions were isolated and identified in the present study. Isolate C. globosum F211.UMNG-derived extract inhibits the growth of F. oxysporum, possibly by at least three molecules having different modes of action, implying its possible application in control schemes of F. oxysporum27. A confirmation of the results through in vivo testing is required, involving endophyte C. globosum and purifying the identified compounds for evaluating their ability in the control of the disease caused by F. oxysporum.
Ethical responsibilities
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Funding
The present work was financed by Vicerrectoría de Investigaciones at UMNG through the project INV-CIAS-1472.
Conflict of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
Authors thank Universidad Militar Nueva Granada (UMNG) for the financial support. The present work is a product derived from the Project CIAS-1472 financed by Vicerrectoría de Investigaciones at UMNG.
1. Baayen RP, Bonants PJM, Verkley G, Carroll GC, Van Der Aa HA, De Weerdt M, van Brouwershaven IR, Schutte GC, Maccheroni W, de Blanco CG, Azevedo JL. Nonpathogenic isolates of the citrus black spot fungus, Guignardia citricarpa, identified as a cosmopolitan endophyte of woody plants, G. mangiferae (Phyllosticta capitalensis). Phytopathology. 2002;92:464-77. [ Links ]
2. Bara R, Aly AH, Pretsch A, Wray V, Wang B, Proksch P, Debbab A. Antibiotically active metabolites from Talaromyces wortmannii, an endophyte of Aloe vera. J Antibiot. 2013;66:491-3. [ Links ]
3. Barnett H, Hunter B. Illustrated genera of imperfect fungi. 4th ed. Minnesota: APS Press; 1998. p. 8-34. [ Links ]
4. Bittleston LS, Brockmann F, Wcislo W, Van Bael SA. Endophytic fungi reduce leaf-cutting ant damage to seedlings. Biol Lett. 2011;7:30-2. [ Links ]
5. Borges WS, Mancilla G, Guimaraes DO, Durán-Patrón R, Collado IG, Pupo MT. Azaphilones from the endophyte Chaetomium globosum. J Nat Prod. 2011;74:1182-7. [ Links ]
6. Davis EC, Franklin JB, Shaw AJ, Vilgalys R. Endophytic Xylaria (Xylariaceae) among liverworts and angiosperms: phylogenetics, distribution, and symbiosis. Am J Bot. 2003;90:1661-7. [ Links ]
7. de Lima Fávaro LC, de Souza Sebastianes FL, Luiz Araujo W. Epicoccum nigrum P16, a sugarcane endophyte, produces antifungal compounds and induces root growth. PLoS ONE. 2012;7:e36826. [ Links ]
8. Demers JE, Gugino BK, Jiménez-Gasco M. Highly diverse endophytic and soil Fusarium oxysporum populations associated with field-grown tomato plants. Appl Environ Microbiol. 2015;81:81-90. [ Links ]
9. Dewanjee S, Gangopadhyay M, Bhattacharya N, Khanra R, Dua TK. Bioautography and its scope in the field of natural product chemistry. J Pharm Anal. 2015;5:75-84. [ Links ]
10. El-Nagerabi SA, Elshafie AE, Alkhanjari SS. Endophytic fungi associated with endogenous Boswellia sacra. Biodiversitas. 2014;15:24-30. [ Links ]
11. Ezra D, Hess WM, Strobel GA. New endophytic isolates of Muscodor albus, a volatile-antibiotic-producing fungus. Microbiology. 2004;150:4023-31. [ Links ]
12. Gompper ME, Hoylman A. Grooming with Trattinnickia resin: possible pharmaceutical plant use by coatis in Panama. J Trop Ecol. 1993;9:533-40. [ Links ]
13. Hall TA. BioEdit: an important software for molecular biology. GERF Bull Biosci. 2011;2:60-1. [ Links ]
14. Hanlin RT. Combined keys to illustrated genera of ascomycetes, vols. 1 and 2. Minnesota: APS Press; 1998. [ Links ]
15. Hoepfner D, McNamara CW, Lim CS, Studer C, Riedl R, Aust T, Winzeler EA. Selective and specific inhibition of the Plasmodium falciparum lysyl-tRNA synthetase by the fungal secondary metabolite cladosporin. Cell Host Microbe. 2012;11: 654-63. [ Links ]
16. Hwang EI, Yun BS, Kim YK, Kwon BM, Kim HG, Lee HB, Bae KS, Kim SU. Chaetoatrosin A, a novel chitin synthase II inhibitor produced by Chaetomium atrobrunneum F449. J Antibiot. 2000;53:248-55. [ Links ]
17. Joel EL, Bhimba BV. Evaluation of secondary metabolites from mangrove associated fungi Meyerozyma guilliermondii. Alexandria J Med. 2013;49:189-94. [ Links ]
18. Kanchiswamy CN,Malnoy M, Maffei ME. Bioprospecting bacterial and fungal volatiles for sustainable agriculture. Trends Plant Sci. 2015;20:206-11. [ Links ]
19. Keller N, Turner G. Fungal secondary metabolism methods and protocols. Totowa, NJ: Humana Press; 2012. [ Links ]
20. Kidane EG, Laing MD. Integrated control of Fusarium wilt of banana (Musa spp.). International Conference on Banana and Plantain in Africa, Harnessing International Partnerships to Increase Research Impact. Acta Hort. 2010;879: 315-21. [ Links ]
21. Kiffer E, Morelet M. Thedeuteromycetes, mitosporic fungi: classification and generic keys. Enfield, NH: Science Publishers; 2000. [ Links ]
22. Kjer J, Wray V, Edrada-Ebel R, Ebel R, Pretsch A, Lin W, Proksch P. Xanalteric acids I and II and related phenolic compounds from an endophytic Alternaria sp. isolated from the mangrove plant Sonneratia alba. J Nat Prod. 2009;72:2053-7. [ Links ]
23. Kusari S, Singh S, Jayabaskaran C. Rethinking production of Taxol (paclitaxel) using endophyte biotechnology. Trends Biotechnol. 2014;32:304-11. [ Links ]
24. Liu Y, Chen S, Liu Z, Lu Y, Xia G, Liu H, She Z. Bioactive metabolites from mangrove endophytic fungus Aspergillus sp.16-5B. Mar Drugs. 2015;13:3091-102. [ Links ]
25. Macías-Rubalcava ML, Hernández-Bautista BE, Oropeza F, Duarte G, González MC, Glenn AE, Hanlin R, Anaya AL. Allelochemical effects of volatile compounds and organic extracts from Muscodor yucatanensis, a tropical endophytic fungus from Bursera simaruba. J Chem Ecol. 2010;36: 1122-31. [ Links ]
26. McWilliam H, Li W, Uludag M, Squizzato S, Park YM, Buso N, Cowley AP, Lopez R. Analysis Tool Web Services from the EMBL-EBI. Nucleic Acids Res. 2013;41:W597-600. [ Links ]
27. Michielse CB, Rep M. Pathogen profile update: Fusarium oxysporum. Mol Plant Pathol. 2009;10:311-24. [ Links ]
28. Morgulis A, Coulouris G, Raytselis YMadden TL, Agarwala R, Schäffer AA. Database indexing for production Mega BLAST searches. Bioinformatics. 2008;15:1757-64. [ Links ]
29. Murali TS, Suryanarayanan TS, Geeta R. Endophytic Phomopsis species: host range and implications for diversity estimates. Can J Microbiol. 2006;52:673-80. [ Links ]
30. Muroga Y, Yamada T, Numata A, Tanaka R. Chaetomugilins I-O, new potent cytotoxic metabolites from a marine-fish-derived Chaetomium species. Stereochemistry and biological activities. Tetrahedron. 2009;65:7580-6. [ Links ]
31. Oerke EC. Crop losses to pests. J Agric Sci. 2006;144:31-43. [ Links ]
32. Oka H, Asahi KI, Morishima H, Sanada M, Shiratori K, limura Y, Sakurai T, Uzawa J, Iwadare S, Takahashi N. Differanisole A, a new differentiation inducing substance. J Antibiot. 1985;38:1100-2. [ Links ]
33. Okane I, Srikitikulchai P, Tabuchi Y,Sivichai S, Nakagiri A. Recognition and characterizatio of four Thai xylaria-ceous fungi inhabiting various tropical foliages as endophytes by DNA sequences and host plant preference. Mycoscience. 2012;53:122-32. [ Links ]
34. Park JH, Choi GJ, Jang KS, Lim HK, Kim HT, Cho KY, Kim JC. Antifungal activity against plant pathogenic fungi of chaetoviridins isolated from Chaetomium globosum. FEMS Microbiol Lett. 2005;252:309-13. [ Links ]
35. Pimentel MR, Molina G, Dionisio AP, Marostica Junior MR, Pastore GM. The use of endophytes to obtain bioactive compounds and their application in biotransformation process. Biotechnol Res Int. 2011, 576286. [ Links ]
36. Qin JC, Gao JM, Zhang YM, Yang SX, Bai MS, Ma YT, Laatsch H. Polyhydroxylated steroids from an endophytic fungus, Chaetomium globosum ZY-22 isolated from Ginkgo biloba. Steroids. 2009;74:786-90. [ Links ]
37. Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406-25. [ Links ]
38. Siani AC, Nakamura MJ, Tappin MRR, Monteiro SS, Guimaraes AC, Ramos MFS. Chemical composition of South American Burseraceae non-volatile oleoresins and preliminary solubility assessment of their commercial blend. Phytochem Anal. 2012;23:529-39. [ Links ]
39. Song F, Wu SH, Zhai YZ, Xuan QC, Wang T. Secondary metabolites from the genus Xylaria and their bioactivities. Chem Biodivers. 2014;11:673-94. [ Links ]
40. Sunayana N, Prakash HS. Fungal endophytes of Boswellia Serrata Roxb.(Burseraceae), a medicinal tree species. IOSRJ Pharm Biol Sci. 2012;1:1-5. [ Links ]
41. Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725-9. [ Links ]
42. Tomoda H, Matsushima C, Tabata N, Namatame I, Tanaka H, Bamberger MJ, Omura S. Structure-specific inhibition of cholestery ester transfer protein by azaphilones. J Antibiot. 1999;52:160-70. [ Links ]
43. Violante IMP, Hamerski L, Garcez WS, Batista AL, Chang MR, Pott VJ, Garcez FR. Antimicrobial activity of some medicinal plants from the cerrado of the central-western region of Brazil. Braz J Microbiol. 2012;43:1302-8. [ Links ]
44. Wang S, Li XM, Teuscher F, Li DL, Diesel A, Ebel R, Proksch P, Wang BG. Chaetopyranin, a benzaldehyde derivative, and other related metabolites from Chaetomium globosum, an endophytic fungus derived from the marine red alga Polysiphonia urceolata. J Nat Prod. 2006;69:1622-5. [ Links ]
45. Wang X, Radwan MM, Tarawneh AH, Gao J, Wedge DE, Rosa LH, Cutler H, Cutler SJ. Antifungal activity against plant pathogens of metabolites from the endophytic fungus Cladosporium cladosporioides. J Agric Food Chem. 2013;61: 4551 -5. [ Links ]
46. White TJ, Bruns T, Lee SJWT, Taylor JW. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. San Diego, CA: Academic Press Inc.; 1989. p. 315-22. [ Links ]
47. Yang X, Huang L, Li HY, Yang DF, Li ZZ. Two new compounds from the plant endophytic fungus Pestalotiopsis versicolor. J Asian Nat Prod Res. 2015;17:333-7. [ Links ]
48. Zaher AM, Makboul MA, Moharram AM, Tekwani BL, Calderón AI. A new enniatin antibiotic from the endophyte Fusarium tricinctum Corda. J Antibiot. 2015;68:197-200. [ Links ]
49. Zhang G, Zhang Y,Qin J, Qu X, Liu J, Li X, Pan H. Antifungal metabolites produced by Chaetomium globosum No. 04, an endophytic fungus isolated from Ginkgo biloba. Indian J Microbiol. 2013;53:175-80. [ Links ]
50. Zhang Q, Li HQ, Zong SC, Gao JM, Zhang AL. Chemical and bioactive diversities of the genus Chaetomium secondary metabolites. Mini Rev Med Chem. 2012;12:127-48. [ Links ]














