Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Revista argentina de microbiología
versão impressa ISSN 0325-7541versão On-line ISSN 1851-7617
Rev. argent. microbiol. vol.50 no.3 Ciudad Autónoma de Buenos Aires set. 2018
http://dx.doi.org/10.1016/j.ram.2017.08.002
BRIEF REPORT
https://doi.org/10.1016/j.ram.2017.08.002
Streptococcus agalactiae bacteremia in non-pregnant adult patients at two teaching hospitals
Bacteriemia por Streptococcus agalactiae en pacientes adultos y mujeres no embarazadas de dos hospitales universitarios
Emanuel J. Saada,b,*, Diego F. Baenasa, Cecilia S. Boisseauc, Mercedes J. Garcíaa, Silvana A. Núñeza, Pablo E. Sanchezc, Domingo C. Balderramob,d, Daniela Hernándeze, Juan P. Caeirob,c
a Internal Medicine Department, Hospital Privado Centro Médico de Córdoba, Naciones Unidas N° 346, CP: 5016, Córdoba, Argentina
b Instituto Universitario de Ciencias Biomédicas de Córdoba, Naciones Unidas N° 400, CP: 5016, Córdoba, Argentina
c Infectious Disease Department, Hospital Privado Centro Médico de Córdoba, Naciones Unidas N° 346, CP: 5016, Córdoba, Argentina
d Gastroenterology Department, Hospital Privado Centro Médico de Córdoba, Naciones Unidas N° 346, CP: 5016, Córdoba, Argentina
e Microbiology Department, Hospital Privado Centro Médico de Córdoba, Naciones Unidas N° 346, CP: 5016, Córdoba, Argentina
Received 20 February 2017; accepted 9 August 2017
Available online 7 December 2017
*Corresponding author.
E-mail address: emanuelsaad@hotmail.com (E.J. Saad).
0325-7541/© 2017 Asociacion Argentina de Microbiología. Published by Elsevier España, S.L.U. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Abstract
Streptococcus agalactiae or group B streptococcus (GBS) is a frequent pathogen in immunocompromised adults. The aim of this study was to determine the relative frequency, clinical presentation, antimicrobial susceptibility profile, and risk factors associated with GBS bacteremia in non-pregnant adult patients. We conducted a retrospective analysis of blood cultures performed in two hospitals between the years 2009-2013. From 1110 bacteremia episodes, 13 were caused by GBS, all of which were susceptible to ampicillin. GBS bacteremia was more frequent in females and in patients older than 60 years of age. The most frequent comorbidities were chronic kidney disease, cardiac failure and neoplasia. History of appendectomy was detected in 53.8% of the patients, being the most relevant comorbidity for GBS bacteremia in the multivariate analysis (OR 4.13, p = 0.012). The main presentations were primary bacteremia and soft tissue infection. GBS bacteremia was infrequent in our institution, and a history of appendectomy might be related to bacteremia occurrence.
KEYWORDS
Adults; Bacteremia; Streptococcus agalactiae
Resumen
Streptococcus agalactiae o estreptococo del grupo B (SGB), es un patógeno frecuente en adultos inmunocomprometidos. El objetivo de este trabajo fue determinar la frecuencia relativa, formas de presentación, susceptibilidad antimicrobiana y factores de riesgo asociados a la bacteriemia por SGB en pacientes adultos y mujeres no embarazadas. Se realizó un análisis retrospectivo de hemocultivos en 2 hospitales entre 2009-2013. De un total de 1.110 episodios de bacteriemia, 13 fueron causadas por SGB, siendo todos los aislamientos sensibles a ampicilina. Fue más frecuente en mujeres y en pacientes mayores de 60 años. Las comorbilidades más frecuentes fueron enfermedad renal crónica, insuficiencia cardíaca y neoplasias. El 53,8% de los casos tenía antecedente de apendicectomía previa, siendo la comorbilidad más relevante según el análisis multivariado (OR: 4,13; p = 0,012). Se presentaron principalmente como bacteriemia primaria e infección de tejidos blandos. La bacteriemia por SBG fue infrecuente en nuestro medio y el antecedente de apendicectomía podría relacionarse al desarrollo de la misma.
PALABRAS CLAVE
Adulto; Bacteriemia; Streptococcus agalactiae
Streptococcus agalactiae or group B streptococcus (GBS) was initially reported as a human pathogen in 1938 following three cases of fatal postpartum sepsis. Since then, it has been identified as a causal agent of severe disease in pregnant women, postpartum women, and infants. In the 1990s, the implementation of GBS screening during pregnancy and intrapartum chemoprophylaxis resulted in a significant reduction of neonatal mortality to less than 5%15.
An increase in the incidence of GBS invasive infection in adults has been observed, being bacteremia the main clinical presentation. The reasons of this epidemiological shift have not been clearly explained. It has been postulated that advancing age, diabetes mellitus, neoplastic disease, and a history of organ transplantation represent the main risk factors for GBS invasive disease in adult patients. However, there are several cases that have not been identified as associated risk factors3,12.
GBS virulence factors allow the colonization and invasion of the epithelial barriers. The disruption of main reservoir sites (such as the gastrointestinal and the genitourinary tracts) as well as the interaction with the immune system, could contribute to the development of invasive disease associated to GBS.
The aim of this study was to determine the relative frequency and risk factors associated with GBS bacteremia in non-pregnant adult patients. Furthermore, the clinical presentation and the antimicrobial susceptibility profile of GBS were also analyzed.
The study was conducted in two polyvalent and university hospitals from Córdoba, Argentina (Hospital Privado Centro Médico de Córdoba and Hospital Raúl Ferreyra). Both centers share the same Department of Microbiology. This study has been approved by the Health Ethics Committee "Comité Institucional de Ética de Investigación en Salud" of "Hospital Privado Centro Médico de Córdoba".
We performed a retrospective study that evaluated all positive blood cultures (BC) from the database of the Department of Microbiology from April, 2009 to August, 2013 in patients older than 18 years of age. Pregnant and postpartum women were excluded. We compared cases with positive BC for GBS with all other etiologies in the same period. The following variables were compared between both groups: age, sex, place of infection acquisition, clinical presentation, associated risk factors and mortality. The following comorbidities were analyzed as possible risk factors: peripheral artery disease, cardiac failure, ischemic heart disease, kidney disease, discriminated renal replacement therapy, diabetes mellitus, neoplastic disease, history of smoking, chronic obstructive pulmonary disease, asthma, neurological disease, history of solid organ or bone marrow transplantation, current chemotherapy status, corticosteroids (equal or higher than 20mg/day of prednisone for more than 20 days) or immunosuppressive drugs, HIV/AIDS infection, asplenia, immunoglobulin deficiency, history of appendectomy, and diagnosis of febrile neutropenia during the episode. Each comorbidity was considered according to the current definitions. The resistance profile of GBS was evaluated.
GBS bacteremia was defined as positive BC for GBS in at least one blood sample. Community-acquired infection was defined as positive BC obtained within the first 48 h of hospitalization, not existing during that period any healthcare activity that may have caused it. We defined hospital-acquired bacteremia as the infection acquired by an inpatient admitted for a cause other than bloodstream infection after 48 h of hospitalization, or the infection acquired between 48 and 72 h before discharge. Healthcare-associated bacteremia was considered an infection secondary to a diagnostic or therapeutic procedure performed in an outpatient setting (urinary catheter, intravenous catheter, chronic hemodialysis, and peritoneal dialysis), and those bacteremias acquired by inpatients admitted to geriatric and long-term care homes. The probable site of the infection was also determined according to each patient's medical record. Those cases in which there was isolation of a microorganism in BC without identification of the focus, were defined as bacteremia without focus.
The Department of Microbiology used the continuous monitoring blood culture system using fluorescent-sensor technology (BACTEC 9240, Becton Dickinson). Identification and antimicrobial susceptibility testing was performed using the automated method Vitek 2 system (bioMerieux). GBS was identified with GP ID cards and the antimicrobial susceptibility testing was performed with AST-577 cards. The D test method was used for the detection of inducible clindamycin resistance (Kirby-Bauer).
Continuous variables are expressed as means and standard deviations, or as medians and ranges on the basis of their homogeneity. Categorical variables are expressed as numbers and percentages. To assess the association between individual risk factors and GBS invasive infection, we used independent the t or chi-square tests. Thereafter, all variables that were significant in the univariate analysis with a p value <0.15 were considered for a multivariate logistic regression analysis so that independent risk factors for GBS invasive infection could be determined. The associations are presented as odds ratios (ORs) and 95% confidence intervals (CIs). A 2-sided probability value <0.05 was considered to be significant. Statistical analysis was performed using the SPSS 17.0 statistical package (SPSS, Inc., Chicago, IL).
During the study period a total of 1110 bacteremia episodes were identified. GBS was present in 13 episodes of bacteremia (1.18%). The mean age of the 13 patients with GBS bacteremia was 66 years (range: 22-94 years), and 76.9% were older than 60 years of age. We detected 8 cases of GBS bacteremia in women (61.5%). Community-acquired GBS bacteremia was diagnosed in 9 patients (69.2%), nosocomial bacteremia in 3 (23.1%) and healthcare-associated bacteremia in one patient undergoing hemodialysis (7.7%). In two cases (15.4%), polymicrobial isolation was reported, being GBS associated with Stenotrophomonas maltophilia in one case and Staphylococcus aureus in another case.
The main clinical presentations were bacteremia without apparent focus and soft tissue infection (5 cases each focus, 38.5%). We registered one case of native aortic valve endocarditis confirmed by transesophageal echocardiogram, in a patient who had previously contracted community-acquired pneumonia. Furthermore, there was one case of urinary tract infection and another of abdominal infection. Three patients were admitted to the intensive care unit for septic shock and one of them died (bacteremia without focus).
The most frequent risk factors associated with GBS bacteremia were chronic kidney disease (30.8%), cardiac failure (30.8%), history of neoplasia (30.8%), history of ischemic heart disease (23.1%), and diabetes mellitus (15.4%) (Table 1). Nine (69.2%) patients presented two or more comorbidities. Only one patient did not have any of the comorbidities associated with this infection. Interestingly, 53.8% of the patients had a previous history of appendectomy. Table 1 shows the comorbidities in the control group of patients. The multivariate analysis revealed that the history of appendectomy (OR 4.13, 95% CI 1.37-12.45, p = 0.012) was the only independent comorbidity for GBS bacteremia. History of cervix carcinoma showed a borderline association as risk factor (OR 8.67, 95% CI 0.98-76.58, p = 0.052).
Table 1 Clinical characteristics of patients with GBS bacteremia vs. bacteremia due to other etiologies
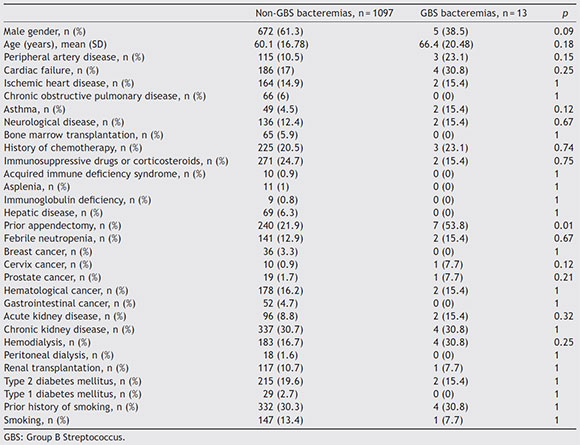
Mortality associated with GBS bacteremia differ from that associated with bacteremia caused by other agents, but differences are not significant (7.7% vs. 24.1%, p = 0.32).
With regard to the profile of antimicrobial susceptibility, all the GBS strains isolated during the study period were susceptible to ampicillin. We observed resistance to erythromycin and clindamycin in 2 (23%) of the diagnosed cases.
In the last two decades, the implementation of GBS screening and risk-based intrapartum chemoprophylaxis, significantly reduced neonatal and maternal mortality. However, a remarkable increase in GBS invasive infections in immunocompromised non-pregnant adults has been observed, this subgroup of patients accounting for 66% of the total number of cases12. The clinical presentation of GBS invasive infection as bacteremia has increased in the last few years. This has been evidenced by a recent study performed in Latin America that evaluated all cases of GBS infection during 17 years, finding a difference in the percentage of bacteremia associated with GBS between two periods (37%o in 1994-2001 vs. 60%o in 2004-2012). Moreover, when the episodes of bacteremia from the first study period were analyzed, 35.3% of the isolates were obtained from adults, whereas in the second period this subgroup accounted for 70.2% of the cases3.
In our study, we observed that GBS invasive disease associated with bacteremia was infrequent within the group of bacteremias caused by any bacterial agent. Similarly to other series, the main clinical presentation of GBS bacteremia was bacteremia without apparent focus and bacteremia associated with soft tissue disease3,13. The frequency of GBS endocarditis was low in our series, which included only one case (7.7%) with involvement of the aortic valve. In a previous report, the mitral valve was the most frequently affected valve in GBS endocarditis (mitral valve 48% vs. aortic valve 29%)10. In our study, 76.9% of the patients were older than 60 years, according to different works found in the literature, in which the majority of the patients suffering from GBS bacteremia in the last decades were older3,13. Similarly to other studies (5-25%), mortality from GBS bacteremia in our study was low (7.7%)5,12.
Different comorbidities, such as chronic kidney disease, cardiac failure, ischemic heart disease, neoplasia, and diabetes mellitus5, have been associated with GBS. In most studies from Europe, USA, and Latin America, diabetes was the most relevant comorbidity3,9. In our study the history of appendectomy was the only independent factor, being present in more than half of the cases. Although the pathophysiological mechanisms that could be related to appendectomy are not clearly known, some points should be considered. The main reservoir of GBS in humans is the lower gastrointestinal tract4. The appendix is isolated from the main flow of the digestive tract and has the biggest proportion of gut-associated lymphoid tissue (GALT), which would offer protection against local pathogens14. For these reasons, the appendix constitutes a "safe-house" for a great variety of germ colonies in biofilms representing one of the main enteric reservoirs6,8,11,14. In other scenarios, it has also been observed that the appendix could reduce the risk of recurrence in Clostridium difficile infections6,7,11. Moreover, it has been noted that appendectomy has a moderating effect on the inflammatory immune responses of the gut, since it has been described that appendectomized patients would have a lower risk of developing ulcerative colitis12.
GBS has uniformly remained susceptible to ampicillin for a long time. However, during the last years some strains resistant to erythromycin and clindamycin were isolated, and there are few cases of GBS not susceptible to penicillin3. In our study, all the strains were susceptible to ampicillin, and the percentage of clindamycin and erythromycin resistance was low.
Although our study has the limitations of a retrospective analysis, such as the partial collection of information, we were able to analyze the associated comorbidities of all the episodes of bacteremia diagnosed during the study period. Similarly, we analyzed the risk factors of the control group during the same study period.
In conclusion, GBS bacteremia was rare in non-pregnant adults in our institution, although patients with this diagnosis presented multiple comorbidities. The history of appendectomy was the only different comorbidity related to GBS bacteremia compared with other etiologies. The most frequent clinical presentation was bacteremia without apparent focus and soft tissue infection. GBS was susceptible to ampicillin and had low resistance to erythromycin and clindamycin. Further studies are needed to confirm our findings and evaluate preventive strategies for GBS bacteremia in the adult subgroup of patients.
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors thank Claudio Aviega and Cesar Romero for their technical assistance.
1. Andersson RE, Olaison G, Tysk C, Ekbom A. Appendectomy and protection against ulcerative colitis. N Engl J Med. 2001;344:808-14. [ Links ]
2. Bolin TD, Wong S, Crouch R, Engelman JL, Riordan SM. Appendicectomy as a therapy for ulcerative proctitis. Am J Gastroenterol. 2009;104:2476-82. [ Links ]
3. Crespo-Ortiz Mdel P Castaneda-Ramirez CR, Recalde-Bolanos M, Vélez-Londono JD. Emerging trends in invasive and noninvasive isolates of Streptococcus agalactiae in a Latin American hospital: a 17-year study. BMC Infect Dis. 2014;14:428. [ Links ]
4. Dillon HC Jr, Gray E, Pass MA, Gray BM. Anorectal and vaginal carriage of group B streptococci during pregnancy. J Infect Dis. 1982;145:794-9. [ Links ]
5. Eskandarian N, Neela V, Ismail Z, Puzi SM, Hamat RA, Desa MN, Nordin SA. Group B streptococcal bacteremia in a major teaching hospital in Malaysia: a case series of eighteen patients. Int J Infect Dis. 2013, 17e777-80. [ Links ]
6. Guinane CM, Tadrous A, Fouhy F Ryan CA, Dempsey EM, Murphy B, Andrews E, Cotter PD, Stanton C, Ross RP. Microbial composition of human appendices from patients following appendectomy. MBio. 2013;4:1-6. [ Links ]
7. Im GY, Modayil RJ, Lin CT, Geier SJ, Katz DS, Feuerman M, Gren-dell JH. The appendix may protect against Clostridium difficile recurrence. Clin Gastroenterol Hepatol. 2011;9:1072-7. [ Links ]
8. Jackson HT, Mongodin EF, Davenport KP, Fraser CM, Sandler AD, Zeichner SL. Culture-independent evaluation of the appendix and rectum microbiomes in children with and without appendicitis. PLoS ONE. 2014;9:e95414. [ Links ]
9. Phares CR, Lynfield R, Farley MM, Mohle-Boetani J, Harrison LH, Petit S, Craig AS, Schaffner W, Zansky SM, Gershman K, Stefonek KR, Albanese BA, Zell ER, Schuchat A, Schrag SJ. Active Bacterial Core surveillance/Emerging Infections Program Network Epidemiology of invasive group B streptococcal disease in the United States, 1999-2005. JAMA. 2008;299:2056-65. [ Links ]
10. Pringle SD, McCartney AC, Marshall DA, Cobbe SM. Infective endocarditis caused by Streptococcus agalactiae. Int J Cardiol. 1989;24:179-83. [ Links ]
11. Randal Bollinger R, Barbas AS, Bush EL, Lin SS, Parker W. Biofilms in the large bowel suggest an apparent function of the human vermiform appendix. J Theor Biol. 2007;249:826-31. [ Links ]
12. Schrag SJ, Zywicki S, Farley MM, Reingold AL, Harrison LH, Lefkowitz LB, Hadler JL, Danila R, Cieslak PR, Schuchat A. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med. 2000;342:15-20. [ Links ]
13. Skoff TH, Farley MM, Petit S, Craig AS, Schaffner W, Gershman K, Harrison LH, Lynfield R, Mohle-Boetani J, Zansky S, Albanese BA, Stefonek K, Zell ER, Jackson D, Thompson T Schrag SJ. Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990-2007. Clin Infect Dis. 2009;49:85-92. [ Links ]
14. Smith HF, Fisher RE, Everett ML, Thomas AD, Bollinger RR, Parker W. Comparative anatomy and phylogenetic distribution of the mammalian cecal appendix. J Evol Biol. 2009;22:1984-99. [ Links ]
15. Verani JR, McGee L, Schrag SJ, Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases Centers for Disease Control and Prevention. Prevention o perinatal grou B streptococcal disease revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59:1-36. [ Links ]














