Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista argentina de microbiología
Print version ISSN 0325-7541On-line version ISSN 1851-7617
Rev. argent. microbiol. vol.50 no.4 Ciudad Autónoma de Buenos Aires Dec. 2018
http://dx.doi.org/10.1016/j.ram.2017.11.004
ORIGINAL ARTICLE
https://doi.org/10.1016/j.ram.2017.11.004
Prevalence of Trichomonas vaginalis and Human papillomavirus in female sex workers in Central Veracruz, Mexico
Prevalencia de Trichomonas vaginalis y virus del papiloma humano en mujeres trabajadoras sexuales en el centro de Veracruz, México
Azucena Munoz-Ramireza, Aracely Lopez-Monteonb, Angel Ramos-Ligoniob, Enrique Mendez-Bolainac, Mario R.B. Guapillo-Vargasc,*
a Doctorado en Ciencias Biomédicas, Universidad Veracruzana, Xalapa, Veracruz, Mexico
b LADISER Inmunología y Biología Molecular, Facultad de Ciencias Químicas, Universidad Veracruzana, Orizaba, Veracruz, Mexico
c Laboratorio de Biología Molecular, Facultad de Ciencias Químicas, Universidad Veracruzana, Orizaba, Veracruz, Mexico
Received 28 June 2017; accepted 9 November 2017
Available online 13 March 2018
*Corresponding author.
E-mail address: mguapillo@uv.mx (M.R.B. Guapillo-Vargas).
0325-7541/© 2017 Asociacion Argentina de Microbiología. Published by Elsevier España, S.L.U. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Abstract
Female sex workers (FSWs) have been considered a key population for sexually transTrichomonas mitted infections (STIs); therefore, they are periodically screened as a requirement to obtain a work card. However, there is insufficient epidemiological data on STIs among FSWs in Mexico. The detection of Trichomonas vaginalis is limited to microscopic studies and the molecular screening of Human papillomavirus (HPV) is only done to women 35 years of age and older. The objective of this study was to determine the prevalence of T. vaginalis and HPV infections in FSWs in the city of Orizaba, Veracruz, Mexico. Samples from 105 FSWs were obtained by cervical swab and analyzed. The identification of T. vaginalis and HPV was performed by molecular methods. HPV DNA was identified in 5.71% of the samples with the presence of HPV16, HPV18, and HPV58. A percentage of 25.7% samples were positive for T. vaginalis for optical microscopy and 23.8% for PCR. The results of the study indicate the need to incorporate more sensitive methods for the timely diagnosis of STIs as well as comprehensive health promotion programs directed to the most vulnerable groups among FSWs.
KEYWORDS
Trichomonas vaginalis; Human papillomavirus; Female sex workers; Molecular diagnosis
Resumen
Las mujeres trabajadoras sexuales (MTS) han sido consideradas una población clave para las infecciones de transmisión sexual (ITS), por ello son examinadas periódicamente como requisito para obtener una tarjeta de trabajo. Sin embargo, no existen datos epidemiológicos suficientes sobre las ITS en las MTS en México. La detección de Trichomonas vaginalis se limita a los estudios microscópicos, y el cribado molecular del virus del papiloma humano (Human papillomavirus: HPV) solo se realiza en las mujeres de 35 años o mayores. El objetivo de este estudio fue determinar la prevalencia de T. vaginalis e infecciones por HPV en las MTS de la ciudad de Orizaba, Veracruz, México. Se analizaron 105 muestras de las MTS, obtenidas mediante frotis cervical. La identificación de T. vaginalis y HPV se realizó por métodos moleculares. El ADN del HPV se identificó en el 5,71% de las muestras, con la presencia de HPV16, HPV18 y HPV58. El 25,7% de las MTS fueron positivas para T. vaginalis por microscopia óptica el 23,8% por PCR. Los resultados del estudio indican la necesidad de incorporar métodos más sensibles para el diagnóstico oportuno de ITS y programas integrales de promoción de la salud en los grupos más vulnerables, entre las MTS.
PALABRAS CLAVE
Trichomonas vaginalis; Virus del papiloma humano; Mujeres trabajadoras sexuales; Diagnóstico molecular
Introduction
Sexually transmitted infections (STIs) are a group of conditions, which as its name suggests, are predominantly transmitted by sexual contact. Some of these infections are considered reportable in most countries and often continue to be considerably high especially among sexually active young people of reproductive age18. However, several STIs such as Trichomonas vaginalis also have high prevalence in middle-aged women41. Infections with T. vaginalis and Human papillomavirus (HPV) are the most common STIs. The latter has contributed to a variety of adverse outcomes for both sexes, including its partnership in the acquisition of Human immunodeficiency virus (HIV) and, in some cases, it has had a role in the development of cervical neoplasia, among other obstetrical outcomes28. However, the presence of T. vaginalis frequently leads to false positive results in Pap tests. On the other hand, the persistent infection with several HPV types, known as high-risk or oncogenic viruses, is the most important risk factor for developing cervical cancer6,29. In most countries there are certain groups of people who are especially vulnerable to STIs. Overall, the prevalence of STIs tends to be higher in urban areas than in rural areas, among single people and young adults17. The problem of STIs is poorly understood in Mexico, and there are only reports in some areas of the country9,27. STIs in key populations such as female sex workers (FSWs) have been mostly analyzed in Mexico-US border cities2,32,34. In Mexico, trichomoniasis and HPV infection are diseases that are subject to epidemiological surveillance and mandatory notification to health authorities. In 2016, the Health Secretariat reported 46808 new cases for trichomoniasis in women with a higher incidence in 25 to 29-year-old women and a national incidence rate of 90.36/100 000 female population. The state of Veracruz was ranked in the first national place with 6829 new cases; while 28454 new cases of HPV infections were reported in 2016 with a higher incidence in 45 to 49-year-old women and a national incidence rate of 45.43/100000 women1. The national strategy of cervical cancer primary screening is performed by the Pap test and only 35-64-year-old women are screened for HPV DNA by the hybrid capture method. In this study, we aimed to determine the prevalence of T. vaginalis and HPV in FSWs in the city of Orizaba, Veracruz, using molecular detection techniques starting from cervical swabs.
Materials and methods
Study
The cross-sectional study was conducted on FSWs receiving attention in the clinical laboratories of Health Jurisdiction VII from Orizaba, Veracruz, between January 19th, 2011 and November 10th, 2011. Those who provided written informed consent were enrolled in this study. Information on socio-demographic data (age, country of birth, marital status) reproductive health (number of pregnancies, children, termination of pregnancies, type of contraceptives (hormonal or not) and barrier methods used, smoking habits, time in commercial sex work, reasons for attending the clinic, past history of STIs and/or genitourinary infections were obtained in private, using structured questionnaires applied by one investigator. Criteria for inclusion in this study were the following: belonging to the clinical population and patient's willingness to participate. Exclusion criteria were patient refusal and inability to give informed consent. Full gynecological examinations were conducted. Vaginal fluid specimens were collected using sterile cotton swabs before speculum insertion T. vaginalis detection, and cervical and endocervical samples were collected using cytobrushes for the Pap smear and HPV detection, physiological solution was only used to facilitate the introduction of the speculum. Samples were received in the laboratory of Health Jurisdiction VII, stored at 4°C and transported to the laboratory of immunology and molecular biology for further processing. This study was authorized by the Bioethics Committee of the Facultad de Ciencias Químicas (FCQ-CBE-11-033).
Optical microscopy assays
A thin layer spread on slides was made using a vaginal swab. After making the smears, they were dried and fixed with methanol for 3 min. Smears from each vaginal sample were processed for the Giemsa staining technique, were dyed for 20 min and at the end of the staining were washed with distilled water and finally dried at room temperature for further microscopic observation. Samples were considered positive when at least one trophozoite of T. vaginalis was observed. On the other hand, Pap smears were conducted in the laboratories of Health Jurisdiction VII and interpreted using Bethesda 2001 classification38.
Obtaining DNA
A vaginal swab and a cervical brush of each sample were immersed in 1 ml of phosphate buffer [PBS (137 mM NaCl, 2.7mM KCl, 4.3mM Na2HPO4, 1.4mM KH2PO4, pH 7.4)] and repelleted at 2000 x g for 10min. The supernatant was discarded, and the pellet was frozen at-20°C. DNA was extracted as previously described26, thawed samples were resuspended in 600µl of lysis buffer (1 M Tris, 0.5M EDTA, 10% glucose, and lysozyme 2mg/ml), heated at 80°C for 5 min, and then cooled to room temperature. The samples were RNase-treated (Promega, Madison, Wis.) (0.5mg/ml) for 1 h at 37 °C. Proteins were precipitated with 0.2 N NaOH, 1% sodium dodecyl sulfate, 5M potassium acetate (pH 4.8) for 5 min on ice and then centrifuged for 3 min at 2000 x g. DNA was precipitated with 600 µl of isopropanol and then centrifuged for 3 min at 2000 x g, and then the DNA pellet was washed with 600 µl of 70% ethanol and centrifuged for 3 min at 2000 x g. The DNA pellet was dried, resuspended in 50 µl of 10 mM Tris (pH 7.4), 1mM EDTA (pH 8.0), and heated at 65 °C for 1 h. The presence of genomic DNA was confirmed in each sample by electrophoresis prior to PCR amplification.
PCR for T. vaginalis and HPV
T.vaginalis-specific primers TV3 (5-ATTGTCGAACATTGGTCTTACCCTC-3 ) and TV7 (5-TCTGTGCCGTCTTCAAGTATGC-3 )23, and HPV-specific primers MY09/MY01131, were used for PCR amplification. The PCR mixture consisted of 5 µl of 10x PCR buffer, 4 µl of deoxynucleoside triphosphates (2.5mM each), 0.5µl of each primer pair (10pmol/µl),
0.5 µl of Taq DNA polymerase (Promega) (5U/ml), 10 µl of sample (5-10ng/ml), and 29.5µl of distilled water. Positive and negative controls were included in all PCR runs. The positive control consisted of DNA from ATCC T. vaginalis isolate 30 184 and HPV 43 clone 2B, ATCC 40 339. Negative controls included DNA from Trypanosoma cruzi MHOM/MX/1994/INC-1 strain, PCR mix with primers but no DNA, and human genomic DNA. PCR amplification consisted of 30 cycles of 1 min at 90 °C, 30 s at 60 °C, and 2 min at 72°C for T. vaginalis, and 40 cycles of 60s at 95°C, 60s at 55°C, and 60s at 72°C for HPV. After amplification, there was an additional extension step at 72 °C for 7 min, and then the samples were cooled to 4 °C. Five microliters of amplified product were electrophoresed on 1.8% agarose, 0.5mg/ml ethidium bromide gel, viewed on a UV light box and photographed. Samples containing a 300bp fragment were considered positive for T. vaginalis, and samples containing a 450 bp fragment were considered positive for HPV.
HPV typing by E6 nested multiplex PCR
Typing was performed according to reports by Sotlar et al.39. Briefly, from DNA HPV positive samples, there was a first PCR reaction using consensus primers (GP-E6-3F, GP-E7-5B, GP-E7-6B). The PCR mixture consisted of 5 µl of 10x PCR buffer, 4 µl of deoxynucleoside triphosphates (2.5 mM each), 0.5 µl of each primer pair (15pmol/µl), 0.5 µl of Taq DNA polymerase (Promega) (5U/ml), 10µl of sample (5-10ng/ml), and 29.5 µl of distilled water. PCR amplification consisted of 40 cycles of 1 min at 94°C, 60s at 40°C, and 1 min at 72 °C. After amplification, there was an additional extension step at 72°C for 10min. With the product of the first PCR reaction, a second reaction was performed, using the same reaction mixture but with specific primers for each HPV type, HPV16 (5-CACAGTTATGCACAGAGCTGC-3' and 5-CATATATTCATGCAATGTAGGTGTA-3 ), HPV18 (5-CACTTCACTGCAAGACATAGA-3' and 5-GTTGTGAAATCGTCGTTTTTCA-3), and HPV58 (5-GTAAAGTGTGCTTACGATTGC-3' and 5-GTTGT TACAGGTTACACTTGT-3 ). PCR amplification consisted of an initial denaturation step at 94 °C for 4 min, 35 cycles of 30 s at 94 °C, 30 s at 56 °C, and 45 s at 72 °C. After amplification, there was an additional extension step at 72 °C for 4 min. Five microliters of amplified product were electrophoresed on 1.8% agarose, 0.5mg/ml ethidium bromide gel, viewed on an UV light box, and photographed. Samples containing a 457 bp, 322 pb and/or 224 pb fragment were considered positive for HPV16, HPV18 and HPV58 respectively. Negative controls were included in all PCR runs.
Statistical methods
Frequency distribution of demographic data, characteristics of the population, sexual history and clinical manifestations were analyzed. The relationship between selected risk factors and the prevalence of trichomoniasis and HPV infection were compared using χ2 or the Fisher exact test when appropriate. Ninety-five percent confidence intervals were calculated to evaluate statistically significant differences between the collection methods. The relationship between age and prevalence rate was assessed by the Chi-square test and regression analysis. The GraphPad Prism 6 statistics software was used for the statistical analysis.
Results
Sociodemographic characteristics
The 105 samples were divided into 6 age groups as shown in Table 1, age of the participants ranged from 15 to 64 years old, the group between 21 and 30 years had the largest number of samples with 57 (54.29%); the education level showed that of the total studied population, 29.52% (CI 95% 27.4-32) have completed elementary school, 55.23% (CI 95% 52.33-58.13) have completed secondary education, 11.42% (CI 95% 9.67-13.17) have completed high school education, and 3.80% (CI 95% 3.3-4.3) had no education. In relation to marital status, 48.57% (CI 95% 45.38-51.76) were single, 18.10% (CI 95% 16.16-20.02) were married, and 33.3% (CI 95% 31.11-35.49) reported living together, being divorced or widowed.
Table 1 Socioeconomic characteristics and sexual behavior of FSWs
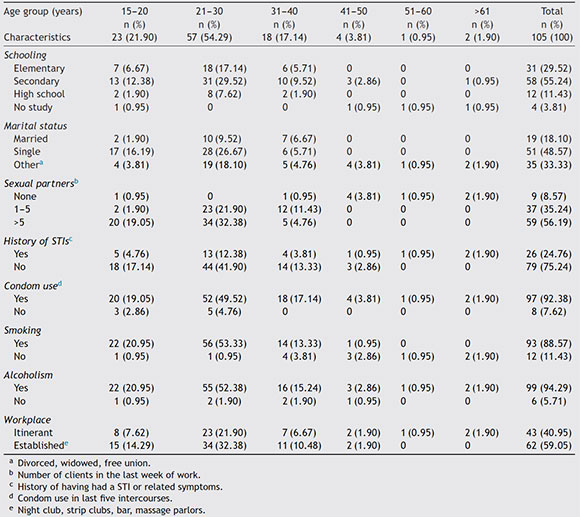
When asked about sexual intercourse during their last week of work, 56.19% (CI 95% 52.59-59.80) reported having intercourse more than five times a week, 35.24% (CI 95% 32.18-38.25) said they had intercourse between 1 and 5 times, 8.57% (CI 95% 7.67-9.47) had no intercourse at all. In order to keep a historical record about the presence of STIs in this study group, they were questioned whether they had ever suffered at least one STI in their lives or if they had related symptoms; 75.23% (CI 95% 71.51-78.95) reported not having had an STI while the remaining 26 participants (24.76%, CI 95% 23.02-26.5) reported having suffered at least one STI; however, they did not reveal its causal agent. Similarly, they were asked whether they regularly used condoms during intercourse, 7.61% (8/105, CI 95% 6.12-9.1) demonstrated inconsistent condom use. In relation to smoking and alcoholism, 88.57%o (93/105, CI 95%o 84.15-92.99) of FSWs were smokers, and 99 of them (94.28%, CI 95% 90.2-98.36) consumed alcohol while performing their activities. Finally, in relation to the work site where they offered their services, 40.95%o FSWs (43/105, CI 95%o 38.45-43.42) answered that they did so on the street, and the remaining 62 (59.05%, CI 95% 55.77-62.31), reported offering their services in established places such as bars, nightclubs, strip clubs, and massage parlors.
Prevalence and variables associated with the presence of risk T. vaginalis and HPV infections
All 105 samples were used to identify the presence of T. vaginalis and HPV by PCR, a parallel sample of the exudate was used to search for trophozoites of T. vaginalis by light microscopy, 25.7%o (27/105, CI 95%o 23.56-27.85) of positive samples were obtained by microscopic analysis for the presence of trophozoites of T. vaginalis (data not shown), moreover, the samples were analyzed by the Pap test for screening of cervical cancer precursor lesions, finding 7.61% (8/105, CI 95% 6.25-8.97) of samples with cytological abnormalities such as atypical squamous cells of undetermined significance (ASC-US) (25.0%, 2/8), low-grade squamous intraepithelial lesions (LSIL) (62.5%, 5/8) and high-grade squamous intraepithelial lesions (HSIL) (12.5%, 1/8).
Molecular diagnosis of T. vaginalis showed an overall prevalence of 23.8% (25/105, CI 95% 21.82-25.78), as shown in Table 2, all positive samples showed a 300 bp amplicon, identifying a mean age of 24.7 years for women positive for T. vaginalis. When we analyzed the presence of HPV genomic material in the 105 samples studied, 5.71% (6/105, CI 95% 4.7-6.72) samples resulted positive for amplification of a 450 bp fragment specific for HPV, as shown in Table 2, with an average age of 27.6 years for women who tested positive for the presence of HPV. Additionally, we performed the genotyping of HPV-positive samples and we observed the presence of high-risk types; identifying HPV16 (16.6%, 1/6), HPV18 and HPV58 (33.3%, 2/6), and HPV16, HPV18 and HPV58 (50.0%, 3/6) as shown in Figure 1. In relation to the diagnosis of T. vaginalis, 25 samples had positive results for two tests; the agreement between the optical microscopy and the molecular diagnostic was very good, (optical microscopy versus PCR k = 0.949 ±0.036, CI 95% 0.879-1.019). Further stratification of FSWs from the municipality of Orizaba showed a significant difference in infection rate (T. vaginalis) according to age (χ2 =64.27, degrees of freedom = 5, p = 0.0001), and HPV (χ2 = 21.85, degrees of freedom = 5, p = 0.0006). Prevalence rate was significantly correlated with age (r2 =0.8178, p = 0.0159, by second-order polynomial regression) for T. vaginalis infection, and (r2 =0.922, p = 0.0076 by second-order polynomial regression) for HPV infection, as shown in Figure 2. On the other hand, the presence of infection by T. vaginalis was significantly associated with educational level (basic education versus higher education) (3% versus 21%, p = 0.0138, CI 95% 0.0875-0.8496, by the Fisher's exact test), but not for HPV infection (p = 0.0765, by the Fisher's exact test), for both infections there was neither association with marital status of participants (p = 0.274 for T. vaginalis infection, p = 0.146 for HPV infection, by Fisher's exact tests), nor with the number of clients per week (p = 0.074 for T. vaginalis infection, p = 0.40 for HPV infection, by Fisher's exact tests). However, there was a significant association between the presence of infection and participants who reported having a history of STIs (p < 0.0001, CI 95% 3.68-16.58, RR = 7.81, for T. vaginalis infection, and p = 0.025, CI 95% 1.28-33.77, RR = 6.58, for HPV infection, by Fisher's exact tests). It was also noted that there was no significant association between condom use (T. vaginalisp = 0.391, HPVp = 0.386, by the Fisher's exact test), alcohol consumption (T. vaginalis p = 1.0, HPV p = 0.303, by the Fisher's exact test), and smoking (T. vaginalis p = 0.726, HPV p = 0.138, by the Fisher's exact test) with the presence of infection. Finally, we observed a significant association between the place of work (street versus established place) and the presence of T. vaginalis infection (p = 0.0023, CI 95% 1.45-6.45, RR = 3.064, by Fisher's exact test) but not for HPV infection (p = 0.224, by the Fisher's exact test).
Table 2 DNA detection of T. vaginalis and HPV by age

Positive FSWs to T. vaginalis, HPV and/or abnormal cytology were referred to gynecological care according to national health guidelines.
Discussion
This study identified the characteristics in the FSWs of the central area of Veracruz, Mexico whose labor-economic activity exposes them to acquiring STIs. Some sociodemographic characteristics of FSWs correspond to those found in other studies in Mexico and some countries in the region, which show that sex work is performed by young women between the ages of 15 and 40, some of them being minors, with low school level, single or in free union and heavy smokers and alcohol consumers24,36.
The region of the study is composed of municipalities with high social and economic inequality, some of them classified as having the lowest index of human development in Mexico. These conditions of poverty and the lack of legislation that guarantee the protection of sexual work, place the FSWs of Veracruz in a vulnerable position30. However, this same regional context excludes them from other phenomena present in border cities, mainly in cities of the US-Mexico border, such as the use of injectable drugs by FSWs and their clients, which increases the risk of acquiring and transmitting HIV, sex tourism and violence associated with the migratory context11,12,42.
FSWs have historically been a key population in the HIV epidemic and strategies have been implemented to reduce the bridge of HIV transmission in sex work. Of the total HIV cases in Mexico, 20% occur in women with an incidence rate of 2.01/100 000 women1. In 2014, the prevalence of HIV among FSWs was 0.67%, which contrasts with that reported in male sex workers, which was 24.1%, with FSWs being the only key population that has decreased their HIV incidence due to the promotion of condom use16,20. In our study, we found a higher percentage of condom use compared to FSWs in Mexico's border cities like Tijuana and Ciudad Juárez44 and Central American countries40. The use of condoms by sex workers shows special patterns according to the regional context, such as free access to condoms, differential condom uses with clients and non-commercial sexual partners, and the negotiation of sexual practices not protected by money4. Therefore, further studies on the perception and coverage of condom use in the FSWs of Veracruz, Mexico are needed.
Trichomoniasis and HPV infection are notifiable diseases in Mexico, however, epidemiological data reported by the Health Secretariat does not represent the real magnitude of these STIs because there is no total coverage of the public health services and because many STIs are asymptomatic. In addition, there is no official data of these STIs in FSWs; therefore, the information available is limited to academic studies conducted in a few cities in Mexico. In our study, we found a prevalence of T. vaginalis of 23.81% in FSWs, which is lower than that shown in cities in the Mexico-US Border Region, which was 35% in the same population type43. In Latin America countries, such as El Salvador, Guatemala, Honduras, Nicaragua and Panama, prevalence was 11%40, while in Peru and Argentina the prevalence of T. vaginalis in FSWs was 9% and 7%, respectively3,8,35. These data show a marked regionalization of T. vaginalis transmission through sex work.
On the other hand, the prevalence of HPV in FSWs was substantially lower than that reported in studies conducted in other cities in Mexico22,33. In HPV positive samples, we identified the presence of HPV16 and HPV18 high risk types, associated with 70% of cervical cancers worldwide5,15,19, and HPV58 high risk type identified with high frequency in studies conducted in women of Mexico, Latin America and China7,13,25. These results suggest that strategies for the prevention of HPV transmission and cervical cancer, including HPV vaccination, condom promotion and primary cervical cancer screening, have been successful in reducing the prevalence of HPV. However, more HPV screening studies including FSWs who work clandestinely or without a registration card are needed to obtain complete evidence.
Mexico's national immunization program includes the application of the vaccine against the HPV16 and HPV-18 high risk types in 11-year-old girls; however, it is necessary to consider the implementation of the second generation HPV vaccine that protects against high-risk HPVs associated with approximately 90% of cervical cancer that includes HPV5810,21, as well as the incorporation of HPV genotyping as part of the strategy of evaluation and control of preventive schemes based on prophylactic vaccines14.
Sex work in Mexico is regulated by each municipal government that requires the periodic screening of some STIs to issue registration licenses to FSWs, however, diagnostic studies are performed in private laboratories that FSWs pay at high cost and often cause extortion and abuse by health authorities, leading to some of the sexual work being carried out clandestinely and without access to STI prevention strategies37.
In conclusion, the results obtained in this study show the need to develop comprehensive health promotion programs for sex workers that include preventive education, campaigns for the timely detection of STIs using sensitive diagnostic methods and the creation of public policies that recognize and protect the Human Rights of FSWs.
Conflict of interest
The authors declare that there is no conflict of interests.
Acknowledgements
Appreciate the support provided by Jurisdicción Sanitaria VII for obtaining samples and David A. Madrigal Serrut for paper revision.
1. Anuario de Morbilidad 1984-2016 [Internet]. México: DGE, Secretaría de Salud; 2017. Available from: http://www.epidemiologia.salud.gob.mx/anuario/html/anuarios.html [updated June 2017; cited 27.06.17].
2. Bazzi AR, Rangel G, Martinez G, Ulibarri MD, Syvertsen JL, Bazzi SA, Roesch S, Pines HA, Strathdee SA. Incidence and predictors of HIV and sexually transmitted infections among female sex workers and their intimate male partners in Northern Mexico: a longitudinal, multilevel study. Am J Epidemiol. 2015;181:723-31. [ Links ]
3. Bologno R, Díaz YM, Giraudo MC, Fernández R, Menéndez V, Brizuela JC, Gallardo AA, Alvarez LA, Estevao Belchior SG. Importance of studying the balance of vaginal content (BAVACO) in the preventive control of sex workers. Rev Argent Microbiol. 2011;43:246-50. [ Links ]
4. Bórquez A, Hallett TB, Gomez GB, Garnett GP. Condom use by female sex workers and their clients in Mexico: who decides and does it matter? Sex Transm Infect. 2011;87:254-6. [ Links ]
5. Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjose S, Bruni L, Tortolero-Luna G, Kjaer SK, Munoz N. Epidemiology and natural history of Human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26:1-16. [ Links ]
6. Braaten KP, Laufer MR. Human papillomavirus (HPV) HPV-related disease, and the HPV vaccine. Rev Obstet Gynecol. 2008;1:2-10. [ Links ]
7. Canche JC, López IR, Suárez NG, Acosta GC, Conde-Ferráez L, Cetina TC, Losa MR. High prevalence and low E6 genetic variability of Human papillomavirus 58 in women with cervical cancer and precursor lesions in Southeast Mexico. Mem Inst Oswaldo Cruz. 2010;105:144-8. [ Links ]
8. Cárcamo CP, Campos PE, García PJ, Hughes JP, Garnett GP, Holmes KK, Peru PREVEN study team. Prevalences of sexually transmitted infections in young adults and female sex workers in Peru: a national population-based survey. Lancet Infect Dis. 2012;12:765-73. [ Links ]
9. Casillas-Vega N, Morfín-Otero R, García S, Llaca-Díaz J, Rodríguez-Noriega E, Camacho-Ortiz A, Ayala-Castellanos Mde L, Mendoza-Olazarán S, Flores-Trevino S, Petersen-Morfín S, Maldonado-Garza HJ, Bosques-Padilla FJ, Garza-González E. Sexually transmitted pathogens, coinfections and risk factors in patients attending obstetrics and gynecology clinics in Jalisco, Mexico. Salud Publica Mex. 2016;58:437-45. [ Links ]
10. Castle PE, MazaM. Prophylactic HPV vaccination: past, present, and future. Epidemiol Infect. 2016;144:449-68. [ Links ]
11. Cepeda A, Nowotny KM. A border context of violence: Mexican female sex workers on the U.S.-Mexico border. Violence Against Women. 2014;20:1506-31. [ Links ]
12. Cepeda A, Nowotny KM, Valdez A. Injecting drug use among Mexican female sex workers on the US-Mexico border. J Ethn Subst Abuse 2015;14:351-63. [ Links ]
13. Chan PK, Zhang C, Park JS, Smith-McCune KK, Palefsky JM, Gio-vannelli L, Coutlée F Hibbitts S, Konno R, Settheetham-Ishida W, Chu TY, Ferrera A, Alejandra Picconi M, De Marco F Woo YL, Raiol T, Piña-Sánchez P Bae JH, Wong MC, Chirenje MZ, Magure T, Moscicki AB, Fiander AN, Capra G, Young Ki E, Tan Y, Chen Z, Burk RD, Chan MC, Cheung TH, Pim D, Banks L. Geographical distribution and oncogenic risk association of Human papillomavirus type 58 E6 and E7 sequence variations. Int J Cancer. 2013;132:2528-36. [ Links ]
14. Choi YJ, Park JS. Clinical significance of Human papillomavirus genotyping. J Gynecol Oncol. 2016;27:e21. [ Links ]
15. Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types i invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003;88:63-73. [ Links ]
16. Comisión Nacional de los Derechos Humanos. Los derechos humanos de las y los trabajadores sexuales que viven con VIH o con sida. México: Comisión Nacional de los Derechos Humanos; 2016 [Online]. http://www.cndh.org.mx/sites/all/doc/cartillas/2015-2016/29-DH-trabaj-sexuales-VIH.pdf [last checked August].
17. Cwikel JG, Lazer T, Press F, Lazer S. Sexually transmissible infections among female sex workers an international review with an emphasis on hard-to-access populations. Sex Health. 2008;5:9-16. [ Links ]
18. Da Ros CT, Schmitt CdaS. Global epidemiology of sexually transmitted diseases. Asian J Androl. 2008;10:110-4. [ Links ]
19. Guan P Howell-Jones R, Li N, Bruni L, de Sanjosé S, Franceschi S, Clifford GM. Human papillomavirus types in 115 789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer. 2012;131:2349-59. [ Links ]
20. Informe nacional de avances en la respuesta al VIH y el sida [Internet]. México: Censida, Secretaría de Salud; 2015. Available from: http://www.censida.salud.gob.mx/descargas/ungass/GARPR_Mx2015.pdf [updated 30.04.15; cited 27.08.17].
21. Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, Moreira ED Jr, Ngan Y, Petersen LK, Lazcano-Ponce E, Pitisut-tithum P Restrepo JA, Stuart G, Woelber L, Yang YC, Cuzick J, Garland SM, Huh W, Kjaer SK, Bautista OM, Chan IS, Chen J, Gesser R, Moeller E, Ritter M, Vuocolo S, Luxembourg A. Broad Spectrum HPV Vaccine Study. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711-23. [ Links ]
22. Juárez-Figueroa LA, Wheeler CM, Uribe-Salas FJ, Conde-Glez CJ, Zampilpa-Mejía LG, García-Cisneros S, Hernández-Avila M. Human papillomavirus: a highly prevalent sexually transmitted disease agent among female sex workers from Mexico City. Sex Transm Dis. 2001;28:125-30. [ Links ]
23. Kengne P Veas F. Vidal N, Rey JL, Cuny G. Trichomonas vaginalis: repeated DNA target for highly sensitive and specific polymerase chain reaction diagnosis. Cell Mol Biol. 1994;40:819-31. [ Links ]
24. Leyva-Flores R, Quintino-Pérez F, Figueroa-Lara A, Cuadra M, Smith D, García C. STI and HIV prevention in female sex workers at border communities in Central America. Salud Publica Mex. 2013;55:S31-8. [ Links ]
25. Liaw KL, Hsing AW, Schiffman MH, You SL, Zhang T, Burk R, Chen CJ. Human papillomavirus types 52 and 58 are prevalent in cervical cancer from Chinese women. Int J Cancer. 1997;73: 775-6. [ Links ]
26. López-Monteon A, Gómez-Figueroa FS, Ramos-Poceros G, Guzmán-Gómez D, Ramos-Ligonio A. Codetection of Trichomonas vaginalis and Candida albicans by PCR in urine samples in a low-risk population attended in a clinic first level in central Veracruz, Mexico. Biomed Res Int. 2013;2013:281892. [ Links ]
27. Magana-Contreras M, Contreras-Paredes A, Chavez-Blanco A, Lizano M, De la Cruz-Hernandez Y, De la Cruz-Hernandez E. Prevalence of sexually transmitted pathogens associated with HPV infection in cervical samples in a Mexican population. J Med Virol. 2015;87:2098-105. [ Links ]
28. Menezes CB, Frasson AP, Tasca T. Trichomoniasis- are we giving the deserved attention to the most common non-viral sexually transmitted disease worldwide? Microb Cell. 2016;3: 404-19. [ Links ]
29. Ogunmodede F, Yale SH, Krawisz B, Tyler GC, Evans AC. Human papillomavirus infections in primary care. Clin Med Res. 2007;5:210-7. [ Links ]
30. Ponce P. L@s guerrer@s de la noche. Lo difícil de la vida fácil. Diagnóstico sobre las dimensiones sociales del trabajo sexual en el Estado de Veracruz. México: Porrúa; 2008. [ Links ]
31. Resnick RM, Cornelissen MT, Wright DK, Eichinger GH, Fox HS, ter Schegget J, Manos MM. Detection and typing of Human papillomavirus in archival cervical cancer specimens by DNA amplification with consensus primers. J Natl Cancer Inst. 1990;82:1477-84. [ Links ]
32. Robertson AM, Syvertsen JL, Ulibarri MD, Rangel MG, Martinez G, Strathdee SA. Prevalenc and correlates of HIV and sexually transmitted infections among female sex workers and their non-commercial male partners in two Mexico-USA border cities. J Urban Health. 2014;91:752-67. [ Links ]
33. Rodríguez-Reyes ER, Quinónez-Pérez JM, Cerda-Flores RM, Saucedo-Cardenas O, Cortés-Gutiérrez EI. Prevalence of HPV in sex workers in Durango, Mexico. Salud Publica Mex. 2005;47:393. [ Links ]
34. Rusch ML, Brouwer KC, Lozada R, Strathdee SA, Magis-Rodríguez C, Patterson TL. Distribution of sexually transmitted diseases and risk factors by work locations among female sex workers in Tijuana, Mexico. Sex Transm Dis. 2010;37:608-14. [ Links ]
35. Salomón MC, Martínez N, Delgado D, González Arra C, Bittar V, González N. Trichomonas vaginalis prevalence in sex workers. Medicina (B Aires). 2011;71:429-31. [ Links ]
36. Semple SJ, Stockman JK, Pitpitan EV, Strathdee SA, Chavarin CV, Mendoza DV, Aarons GA, Patterson TL. Prevalence and correlates of client-perpetrated violence against female sex workers in 13 Mexican cities. PLoS ONE 2015;10:e0143317. [ Links ]
37. Sirotin N, Strathdee SA, Lozada R, Nguyen L, Gallardo M, Vera A, Patterson TL. A comparison of registered and unregistered female sex workers in Tijuana, Mexico. Public Health Rep. 2010;125:101-9. [ Links ]
38. Solomon D, Davey D, Kurman R, Moriarty A, O'Connor D, Prey M, Raab S, Sherman M, Wilbur D, Wright T Jr, Young N, Forum Group Members, Bethesda 2001 Workshop. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114-9. [ Links ]
39. Sotlar K, Diemer D, Dethleffs A, Hack Y, Stubner A, Vollmer N, Menton S, Menton M, Dietz K, Wallwiener D, Kandolf R, Bültmann B. Detection and typing of Human papillomavirus by e6 nested multiplex PCR. J Clin Microbiol. 2004;42:3176-84. [ Links ]
40. Soto RJ, Ghee AE, Nunez CA, Mayorga R, Tapia KA, Astete SG, Hughes JP, Buffardi AL, Holte SE, Holmes KK, Estudio Multicen-trico Study Team. Sentinel surveillance of sexually transmitted infections/HIV and risk behaviors in vulnerable populations in 5 Central American countries. J Acquir Immune Defic Syndr. 2007;46:101-11. [ Links ]
41. Stemmer SM, Adelson ME, Trama JP, Dorak MT, Mordechai E. Detection rates of Trichomonas vaginalis, in different age groups, using real-time polymerase chain reaction. J Low Genit Tract Dis. 2012;16:352-7. [ Links ]
42. Strathdee SA, Lozada R, Semple SJ, Orozovich P Pu M, Staines-Orozco H, Fraga-Vallejo M, Amaro H, Delatorre A, Magis-Rodriguez C, Patterson TL. Characteristics of female sex workers with US clients in two Mexico-US border cities. Sex Transm Dis. 2008;35:263-8. [ Links ]
43. Strathdee SA, Lozada R, Martinez G, Vera A, Rusch M, Nguyen L, Pollini RA, Uribe-Salas F, Beletsky L, Patterson TL. Social and structural factors associated with HIV infection among female sex workers who inject drugs in the Mexico-US border region. PLoS ONE. 2011;6:e19048. [ Links ]
44. Tracas A, Bazzi AR, Artamonova I, Rangel MG, Staines H, Ulibarri MD. Changes in condom use over time among female sex workers and their male noncommercial partners and clients. AIDS Educ Prev. 2016;28:312-24. [ Links ]














