Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Revista argentina de microbiología
versão impressa ISSN 0325-7541versão On-line ISSN 1851-7617
Rev. argent. microbiol. vol.51 no.3 Ciudad Autónoma de Buenos Aires set. 2019
http://dx.doi.org/10.1016/j.ram.2018.10.002
ORIGINAL ARTICLE
https://doi.org/10.1016/j.ram.2018.10.002
Clinical and microbiological characteristics of community-acquired pneumonia associated with Streptococcus pneumoniae in adult patients in Mexico
Características clínicas y microbiológicas de la neumonía adquirida en la comunidad asociada con Streptococcus pneumoniae en pacientes adultos en México
Gabriela Echaniz-Avilesa, Elvira Garza-Gonzálezb, Alma Lucía Román-Manchac, Rayo Morfín-Oterod, Eduardo Rodríguez-Noriegad, Juan Jacobo Ayala-Gaytáne, Claudia Elena Guajardo-Larae, Araceli Soto-Noguerona, Maria Noemí Camalla-Barajasa, Adrián Camacho-Ortizc,*
a Instituto Nacional de Salud Pública, Cuernavaca, Morelos, Mexico
b Servicio de Gastroenterologia y Departamento de Patología Clínica, Hospital Universitario "Dr. José Eleuterio González", Universidad Autónoma de Nuevo León, Monterrey, Nuevo León, Mexico
c Coordinación de Epidemiología Hospitalaria, Hospital Universitario "Dr. José Eleuterio González", Universidad Autónoma de Nuevo León, Monterrey, Nuevo León, Mexico
d Departamento de Infectologia, Hospital Civil Fray Antonio Alcalde, e Instituto de Patología Infecciosa, Guadalajara, Jalisco, Mexico
e Hospital San José Tec de Monterrey, Monterrey, Nuevo León, Mexico
Received 15 March 2018; accepted 9 October 2018
Available online 8 January 2019
*Corresponding author.
E-mail address: acamacho-md@yahoo.com (A. Camacho-Ortiz).
0325-7541/© 2018 Published by Elsevier España, S.L.U. on behalf of Asociación Argentina de Microbiología. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Abstract The aim of this study was to assess the risk factors and clinical and microbiological characteristics of community-acquired pneumonia (CAP) in adult patients in Mexico. Streptococcus pneumoniae classified as the causative agent of CAP in adult patients and patients with invasive S. pneumoniae isolates presented to three tertiary teaching hospitals during the 15-year study period were selected. Serotyping and susceptibility testing were performed for all included isolates. Clinical and demographic data were recorded. A total of 96 patients infected with S. pneumoniae (71 with CAP, 25 with invasive disease) were included. The CAP group involved more males (74.6%) than the invasive disease group (p = 0.03). Head trauma was more common in the CAP group (21.1%) than in the invasive disease group (4.0%; p = 0.03). The most prevalent serotype was 19A, followed by serotypes 3 and 23F. After the introduction of the heptavalent conjugated pneumococcal vaccine (PCV7), the prevalence of included serotypes declined significantly; no such change was found after the introduction of the PCV13 vaccine, including in the prevalence of serotype 19A. Susceptibility to all antimicrobials tested except vancomycin declined over the study period.
In conclusion, head trauma was the most common comorbidity in the CAP group. The most prevalent serotype was 19A. Decreased susceptibility to most antimicrobials tested was observed.
KEYWORDS
Streptococcuspneumoniae; Vaccine; Serotype; Antimicrobial resistance
Resumen El objetivo de este estudio fue evaluar los factores de riesgo y las características clínicas y microbiológicas de la neumonía adquirida en la comunidad (NAC) en pacientes adultos en México. Se seleccionaron pacientes adultos con NAC con Streptococcus pneumoniae como agente causal y pacientes con aislamientos invasivos de S. pneumoniae que concurrieron a tres hospitales de enseñanza de tercer nivel durante el período de estudio de 15 anos (2000-2015). Se realizaron pruebas de serotipificación y sensibilidad con todos los aislados incluidos. Se colectaron los datos clínicos y demográficos. Se incluyeron en total 96 pacientes infectados con S. pneumoniae (71 con NAC y 25 con enfermedad invasiva). El grupo con NAC incluía más varones (74,6%) que el grupo de enfermedad invasiva (p = 0,03). El traumatismo craneoencefálico fue más frecuente en el grupo NAC (21,1%) queen el grupo con enfermedad invasiva (4,0%; p = 0,03). El serotipo más frecuente fue 19A, seguido de los serotipos 3 y 23F. Después de la introducción de la vacuna antineumocócica conjugada heptavalente (PCV7), la prevalencia de los serotipos incluidos en aquella disminuyó significativamente; no sucedió lo mismo después de la introducción de la PCV13, incluso en relación con la prevalencia del serotipo 19A. La sensibilidad a todos los antimicrobianos evaluados, excepto la vancomicina, disminuyó durante el período de estudio.
En conclusión, el traumatismo craneoencefálico fue la comorbilidad más frecuente en el grupo con NAC. El serotipo más frecuente fue el 19A, y se observó disminución de la sensibilidad a la mayoría de los antimicrobianos probados a lo largo del período considerado.
PALABRAS CLAVE
Streptococcuspneumoniae; Vacuna; Serotipo; Resistencia antimicrobiana
Introduction
Streptococcus pneumoniae is among the most frequently isolated pathogens in a variety of infections, including invasive infections such as bacterial meningitis and non-invasive infections such as community-acquired pneumonia (CAP)12 and otitis media10. Thus, vaccination to prevent diseases associated with this microorganism remains one of the most important goals of public health.
CAP is among the most common infectious diseases and a significant cause of mortality and morbidity worldwide. Distinctive bacterial pathogens that cause CAP include S. pneumoniae, and Haemophilus influenzae; the first species being a leading causative agent9,17
As a consequence of extensive antibiotic use, the incidence of S. pneumoniae drug resistance has increased alarmingly; up to 30% of S. pneumoniae strains worldwide are now considered to be multidrug-resistant11,12. Thus, surveillance of the drug resistance of this microorganism in various populations and age groups is important15.
In 2006, the first vaccine for the prevention of infections associated with S. pneumoniae in children was introduced in Mexico. The heptavalent conjugated pneumococcal vaccine (PCV7) included seven serotypes (4, 6B, 9V, 14,18C, 19F, and 23F) and was distributed in lower-income municipalities. It was incorporated into the Mexican universal immunization program in 200815 and was replaced in 2011 by PCV13, which additionally includes serotypes 1, 3, 5, 6A, 7F, and 19A in a 2 +1 schedule6,14.
For the prevention of disease in adults aged >65 years, the pneumococcal polysaccharide vaccine (PPSV 23) is recommended as part of the Mexican universal immunization program since 1983. This vaccine protects against 23 serotypes (1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23Fand 33F).
Epidemiological surveillance of pathogens associated with vaccine-preventable diseases in specific populations and age groups is relevant to detect changes in epidemiology over time and to guide public health policy and the development of control strategies5. Vaccine coverage for a targeted adult population is based on surveys5. Information about circulating S. pneumoniae serotypes in adult patients in Mexico with CAP and the drug susceptibility of these serotypes is scarce, as the Sistema Regional de Vacunas Laboratory Surveillance Program initially focused on children with invasive pneumococcal infections8. Thus, there remains a huge need to describe the clinical and microbiological characteristics of CAP in adult patients in Mexico. This need is supported by the Mexican data requirement for the authorized introduction of PCV13 for adults. The aim of this study was to assess the risk factors, microbiology, and antibiotic resistance of CAP in adult patients in Mexico.
Methods
Design, setting, and patients
Data and samples from patients who presented to three tertiary care hospitals over a 15-year period (January 2000-September 2015) were included in this study. The institutions were the Hospital Universitario Dr. José Eleuterio González, a 500-bed public hospital in the city of Monterrey; the Hospital San José, Tec de Monterrey, a 220-bed private hospital; and the Hospital Civil Fray Antonio Alcalde de Guadalajara, a 1000-bed public hospital.
Cases with clinical and radiological evidence of CAP were detected through a retrospective review of all respiratory samples received from the microbiology laboratory of each hospital. Inclusion criteria were age >18 years, lower respiratory tract (LRT) sample obtained within 48 h of hospital admission, radiographic evidence of new infiltrate, and three or more of the following symptoms: dyspnea, hemoptysis, cough, sputum production, chest pain, fever, headache, and abnormalities on chest auscultation or percussion.
Only patients with radiological changes consistent with pneumonia radiology reports were included. Clinical data, including outcomes, comorbidities, and antibiotic prescriptions, were recovered retrospectively from the patients' hospital records.
Patients with at least one positive S. pneumoniae culture and a clinical diagnosis of pneumonia were included. We also included patients with invasive disease for the comparison of clinical and microbiological data.
Clinically relevant non-invasive CAP isolates were defined as those from sputum, transtracheal aspiration, or bron-choalveolar lavage and others in which S. pneumoniae grew as a single or predominant colony. Sputum samples with >25 leukocytes and <10 epithelial cells per low-power field were processed. The clinical diagnosis of pneumonia was based on (a) positive S. pneumoniae culture, (b) positive radiographic findings, (c) and clinical data indicating pneumonia. Invasive isolates were isolated from blood, cerebrospinal fluid, pleural fluid, and ascites. Specimens were processed according to the guidelines described in the Clinical Microbiology Procedures Handbook.
Patients for whom clinical information was available were included. Sex, age, length of hospital stay, and overall mortality rate, as well as comorbidities and laboratory results, were analyzed. Two different periods were analyzed: the pre-PCV7 period considered from the year 2000 to 2007 and the post-PCV7-PCV13 period from 2008 to 2015.
This work was approved by the Ethical Committee of Universidad de Guadalajara.
Microbiological and susceptibility testing
S. pneumoniae isolates were identified by standard procedures and characteristics, including Gram staining, negative catalase results, sensitivity to optochin, and bile solubility testing. Serotyping was performed using the Quellung reaction and serum from the Statens Serum Institut (Copenhagen, Denmark).
Susceptibility testing was performed by broth microdilution following the guidelines of the Clinical and Laboratory Standards Institute3. Susceptibility to penicillin, erythromycin, chloramphenicol, cefotaxime, trimetho-prim/sulfamethoxazole, and vancomycin was tested. S. pneumoniae ATCC 4919 was used as a control strain.
Statistical analysis
We used conventional dispersion methods for description and non-parametric tests for comparison of the data. The chi-squared test was used to compare dichotomous variables, and the Wilcoxon rank-sum test was used for linear variables. p values <0.05 were considered to be significant.
Analyses were conducted using SPSS software (version 18; SPSS Inc., Chicago, IL, USA).
Results
Isolates, study population, and clinical data
A total of 96 S. pneumoniae isolates with the patients' clinical information were included in the study. Sites of isolation were non-invasive LRT secretions (CAP; n = 66); other non-invasive clinical samples (n = 5) and invasive specimens (n = 25): blood (n = 12), cerebrospinal fluid (n = 6), ascites (n = 1), pleural fluid (n = 5), and brain abscess (n = 1). The CAP group included more men (74.6%) than the invasive group (52%). The median patient age was 43 years (range, 18-94 years), and 66 (68.7%) patients were male.
The length of hospital stay ranged from 0 to 115 days (median, 10 days; Table 1). Head trauma was the most frequent comorbidity in patients with LRT S. pneumoniae (21.12%), and neoplasms were most frequent in patients with invasive strains (12%). Demographic and clinical characteristics are shown in Table 1.
Table 1 Demographic and clinical characteristics of patients with pneumococcal diseases in Mexico

Serotypes and drug susceptibility
All pneumococcal isolates were serotyped with twenty-nine serotypes identified; 4 strains were considered non-typable. Serotype 19A was the most common serotype (19/19.8%), followed by serotypes 3 (11/11.4%) and 23F (7/7.3%).
We detected serotypes included in any of the four available vaccines (3, 4, 6B, 9V, 14, 19F, 23F, 19A, 9N, 11A, 15B, 17F, 20, 22F, and 35B) and 13 serotypes not included in any currently available vaccine (6C, 7C, 13, 15, 15A, 18A, 15C, 16F, 23A, 23B, 24F, 28A, and 38). We also detected serotype 6A, which is included in PCV13 but not in the polyvalent PPSV23 recommended for the adult population (Table 2). Serotypes 1, 5, 7F, 2, 8, 10A, 12F, and 18C, which are included in any of the PCV7, PCV10, PCV13, or PPSV23 vaccines, were not detected.
Table 2 Distribution of pneumococcal serotypes isolated from adult patients according to the prePCV7 and postPCV7-PCV13 period
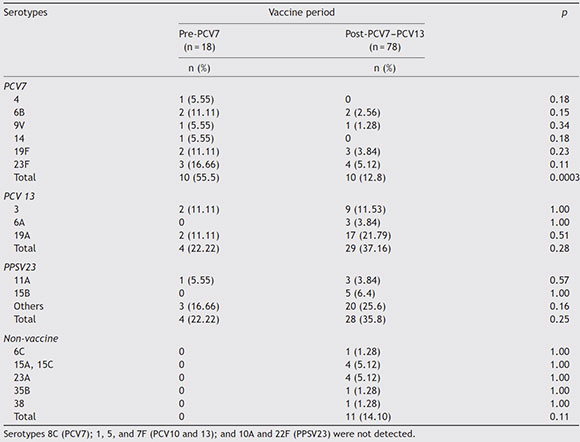
When examined by the pre- and post PCV7 periods, we found 18 pneumococcal isolates in the pre-PCV7 and 78 in the post-PCV7-PCV13 period. The circulation of PCV7 serotypes declined after 2008 (p <0.0003), whereas no reduction was observed for the PCV13 serotypes. A slight but non-significant increase in the circulation of serotype 19A in the post-PCV period was detected (Table 2). There were 4 (22.2%) invasive pneumococcal infections in the pre-PCV7 period and 20 (25.6%) in the post-PCV7-PCV13 period compared to 14 (77.7%) and 58 (74.3%) non-invasive infections, respectively, as shown in Table 3. Isolates not included in any vaccine were only found in the post-PCV7-PCV13 period. No significant differences were found between serotypes causing invasive and non-invasive diseases as shown in Table 3, with serotypes 3 and 19A as the most frequent serotypes in both infections.
Table 3 Distribution of pneumococcal serotypes isolated from invasive and non-invasive diseases by vaccine period in adult population in Mexico

There were 6 pneumococcal meningitis cases reported in the post PCV7 period, and 3 of them showed resistance to penicillin. All of them were susceptible to cefotaxime. For non-meningitis isolates, an important reduction in susceptibility to all antimicrobials except vancomycin (100% in all isolates) during the study period was observed (Table 4), particularly for erythromycin and TMP/SMX. Most of the increase in antimicrobial resistance was due to serotype 19A in the post-PCV7-PCV13 period (data not shown).
Table 4 Drug susceptibility of S. pneumoniae throughout the study period

Discussion
In this study, we aimed to assess the risk factors and microbiological characteristics of CAP in adult patients from three tertiary teaching hospitals in Mexico over a period of 15 years. The most frequent comorbidity in patients with CAP S. pneumoniae was head trauma. This association is difficult to explain, as head trauma is viewed as a risk factor for infection with invasive isolates, rather than CAP isolates. The most frequent comorbidities in patients with non-respiratory strains were neoplasms, which is not surprising because invasive disease (septicemia) caused by S. pneumoniae is a common complication of cancer.
Invasive pneumococcal disease is common in Latin America2,4,16, and the effectiveness of PPSV23 in adult patients varies significantly. Thus, adequate information about circulating serotypes is crucial to define vaccination strategies and aid vaccine development. In this study, we analyzed S. pneumoniae serotype frequencies according to vaccine periods. The changes detected before and after PCV7 and PCV13 were smaller in these adults than in those reported for children, in whom increases in serotypes 19A and 7C and a dramatic reduction in serotype 19A after the introduction of PCV13 have been detected7.
Theoretical serotype vaccine coverage for the pre-PCV7 and the post-PCV7-PCV13 periods were 55.5% and 12.8% for PCV7; 77.8% and 34.6%o for PCV13 and 94.4% and 60.3%o for PPS23 respectively.
The most prevalent serotype in this study during the period before the introduction of PCV7 was 23F, followed by serotypes 6B, 19A, and 19F. After the introduction of PCV7, the frequencies of serotypes 23F and 6B decreased, and serotypes 19Aand 15B predominated. With the introduction of PVC13, serotype 19A continued to be predominant, followed by serotypes 3 and 23A. Although the prevalence of serotype 19A declined after the introduction of PCV13 in children, no such effect was observed in the adults in this study; on the contrary, the frequency of this serotype increased slightly.
Even with the recent changes in dosing regimens for PCV10, an epidemiological impact is apparent in adults, with a reduction in PCV7 serotypes after 2008. In the current study, we detected no reduction in PCV13 or PPSV23 serotypes. This finding has also been reported for the Japanese population15.
After the introduction of PCV7 for children under 2 years, there has been a decrease in the transmission of the included serotypes to adults. This could be due to a herd effect but cannot be concluded with certainty. Serotypes 3 and 19A have low immunogenicity (PCV13), so they do not manage to generate a decrease in colonization of vaccinated children, so they continue to be transmitted to adults. It underscores the need for surveillance of specific populations, particularly adults (including those with CAP), because this population may be a reservoir for different serotypes and the effects seen in children do not reflect protection of older and susceptible community members.
In one of the largest and most recent studies of the antimicrobial susceptibility of S. pneumoniae conducted in Latin America12, 182 S. pneumoniae isolates were found to be predominantly non-susceptible to penicillin (51.6%), with the highest rates of resistance observed in Mexico (84.8%) and Venezuela (81.2%). Similarly, elevated nonsusceptibility rates for ceftriaxone were detected in both countries (21.1-43.7%)12. We identified a notable increase in non-susceptibility to penicillin in our isolates and a steady decrease in susceptibility to cephalosporins; both changes are much greater than previously reported8.
For community meningitis in our population, cefotaxime or ceftriaxone plus vancomycin are used. For NAC, levofloxacin or ceftriaxone plus a macrolide are used. If there is suspicion of penicillin-resistant S. pneumoniae, then vancomycin is added.
Lower susceptibility to trimethoprim/sulfamethoxazole and similar susceptibility to erythromycin have been found in isolates collected from children2,3,4,5.
This study has several limitations. As we were not able to document the patients' immunization status, the influence of vaccination on the selection of individual serotypes remains unclear. In addition, we included sputum samples, which are practical, but imperfect, specimens for the microbiological investigation of CAP. Microscopic examination of sputum quality was performed for all samples, and only mucopurulent material was tested. Nevertheless, sputum is always at risk of contamination with oropharyngeal microbiota. Despite these limitations, national guidelines recommend sputum investigation for patients admitted to hospital with moderate to severe CAP, to aid microbiological diagnosis13.
In this study, more patients with CAP than with invasive disease were male, and head trauma was the most common comorbidity in the CAP group. The most prevalent serotype was 19A, and the proportion of PCV7 serotypes declined after 2008; no reduction in PCV13 serotypes was observed. Decreased susceptibility to most antimicrobials tested was observed. Our results underscore the need for surveillance of the adult population to aid in the control of diseases associated with S. pneumoniae.
Conflict of interest
The authors declare that they have no conflicts of interest.
1. Agudelo CI, Castañeda E, Corso A, Regueira M, Brandileone MC, Brandao AP, Maldonado A, Hormazabal JC, Tamargo I, Echániz-Aviles G, Soto A, Viveros MG, Hernández I, Chamorro G, Weiler N, Sánchez J, Feris JM, Camou T, García G, Spadola E, Payares D, Gabastou JM, Di Fabio JL, Grupo Sireva II. Resistance to nonbeta-lactam antibiotics in the clinical isolates of Streptococcus pneumoniae of children in Latin America. SIREVA II, 2000-2005. Rev Panam Salud Publica. 2009;25:305-13. [ Links ]
2. Castanheira M, Gales AC, Mendes RE, Jones RN, Sader HS. Antimicrobial susceptibility of Streptococcus pneumoniae in Latin America: results from five years of the SENTRY Antimicrobial Surveillance Program. Clin Microbiol Infect. 2004;10:645-51. [ Links ]
3. CLSI. M100-S26. Performance standards for antimicrobial susceptibility testing; twenty-second informational supplement. Wayne, PA: Clinical and Laboratory Standars Institute; 2016. [ Links ]
4. Contreras CL, Verani JR, Lopez MR, Paredes A, Bernart C, Moscoso F, Roldan A, Arvelo W, Lindblade KA, McCracken JP. Incidence of hospitalized Pneumococcal pneumonia among adults in Guatemala, 2008-2012. PLoS ONE. 2015;10:e0140939. [ Links ]
5. Chacon-Cruz E, Rivas-Landeros RM, Volker-Soberanes ML. Early trends in invasive pneumococcal disease in children following the introduction of 13-valent pneumococcal conjugate vaccine: results from eight years of active surveillance in a Mexican hospital. Ther Adv Vaccines. 2014;2:155-8. [ Links ]
6. Daniels N, Valencia-Mendoza A, Gelpi A, Avila MH, Bertozzi S. The art of public health: pneumococcal vaccine coverage in Mexico. Lancet. 2010;375:114-5. [ Links ]
7. Echániz-Avilés G, San Román-Álvarez L, Sánchez-Alemán M, Carnalla-Barajas MN, Soto-Noguerón A. Prevalence of Streptococcus pneumoniae serotype 19A before and after the introduction of the heptavalent conjugate vaccine in Mexico. Salud Publica Mex. 2014;56:266-71. [ Links ]
8. Gabastou JM, Agudelo CI, Brandileone MC, Castaneda E, de Lemos AP, Di Fabio JL, Grupo de Laboratorio de SIREVA II. Characterization of invasive isolates of S. pneumoniae, H. influenzae, and N. meningitidis in Latin America and the Caribbean: SIREVA II, 2000-2005. Rev Panam Salud Publica. 2008;24:1-15. [ Links ]
9. Gentile A, Bardach A, Ciapponi A, Garcia-Marti S, Aruj P, Glu-jovsky D, Calcagno JI, Mazzoni A, Colindres RE. Epidemiology of community-acquired pneumonia in children of Latin America and the Caribbean: a systematic review and meta-analysis. Int J Infect Dis. 2012;16:e5-15. [ Links ]
10. Gómez-Barreto D, de Los Monteros LE, López-Enríquez C, Suarez RR, de la Torre C. Streptococcus pneumoniae serotypes isolated from the middle ear of Mexican children diagnosed with acute otitis media. Salud Publica Mex. 2011;53:207-11. [ Links ]
11. Jones RN, Jacobs MR, Sader HS. Evolving trends in Streptococcus pneumoniae resistance: implications for therapy of community-acquired bacterial pneumonia. Int J Antimicrob Agents. 2010;36:197-204. [ Links ]
12. Jones RN, Guzman-Blanco M, Gales AC, Gallegos B, Castro AL, Martino MD, Vega S, Zurita J, Cepparulo M, Castanheira M. Susceptibility rates in Latin American nations: report from a regional resistance surveillance program (2011). Braz J Infect Dis. 2013;17:672-81. [ Links ]
13. Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM Jr, Musher DM, Niederman MS, Torres A, Whitney CG. American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl. 2:S27-72. [ Links ]
14. Trejo-Valdivia B, Mendoza-Alvarado LR, Palma-Coca O, Hernández-Ávila M, Téllez-Rojo Solís MM. National Survey of Vaccination Coverage (Influenza, pneumococcus and tetanus) in Mexican population of 60 years of age and older. Salud Publica Mex. 2012;54:39-46. [ Links ]
15. Ubukata K, Chiba N, Hanada S, Morozumi M, Wajima T, Shouji M, Iwata S, Invasive Pneumococcal Diseases Surveillance Study Group. Serotype changes an drug resistance in invasive pneumococcal diseases in adults after vaccinations in children, Japan, 2010-2013. Emerg Infect Dis. 2015;21:1956-65. [ Links ]
16. Wiemken TL, Carrico RM, Klein SL, Jonsson CB, Peyrani P, Kelley RR, Aliberti S, Blasi F, Fernandez-Gonzalez R, Lopardo G, Ramirez JA, CAPO Investigators. The effectiveness of the polysaccharide pneumococcal vaccine for the prevention of hospitalizations due to Streptococcus pneumoniae community-acquired pneumonia in the elderly differs between the sexes: results from the Community-Acquired Pneumonia Organization (CAPO) international cohort study. Vaccine. 2014;32: 2198-203. [ Links ]
17. Woodhead M. Community-acquired pneumonia in Europe: causative pathogens and resistance patterns. Eur Respir J Suppl. 2002;36:20s-s27. [ Links ]














