Introduction
Composting can be used to convert livestock farming waste into nutrient-rich biofertilizers which are effectively accessible to plants. Fresh livestock waste contains a large amount of organic nitrogenous compounds (e.g. proteins, nucleic acid and amino acids), which are easily mineralized for producing ammonia. Ammonia produced in composting piles tends to change the pH to alkaline, and subsequently is easily vaporized as free ammonia (NH 3) in the thermophilic stage, which is a major cause of nitrogen loss that reduces the agronomic value of the compost.
As a key process in nitrogen transformation, nitrification is the microbial oxidation of ammonia to nitrate and is significant for keeping ammonia in compost 9. It has been widely assumed that the first step of nitrification is performed almost exclusively by ammonia-oxidizing bacteria (AOB) with all cultivated strains belonging to monophyletic groups within the class Gammaproteobacteria24. Researchers have also discovered another group of ammonia oxidizers, i.e. ammonia-oxidizing archaea (AOA), which can perform ammonia oxidation 15 35. Both groups employ the same functional amoA gene to encode a subunit of the ammonia monooxygenase enzyme in the first step of the nitrification process. Accordingly, molecular techniques using primers targeting the amoA gene have been developed to detect
AOB and AOA in compost since the bacteria grow slowly and some of them cannot be cultured 25.
AOB and AOA have been extensively explored in soil and other ecological niches. However, knowledge about community structure and diversity of AOB and AOA is still limited in compost 11 12 20 9.
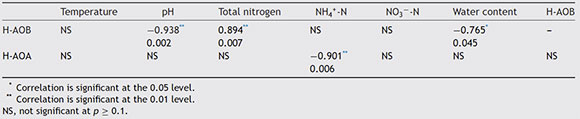
Communities of both AOB and AOA are enormously influenced by environmental factors, such as temperature, pH, water content, various forms of nitrogen and biological factors 6 6. There were some reports focusing on the relationship between the ammonia oxidizer and environmental factors 1. Some researchers found that soil pH had a very significant effect on the community dynamics of AOB and AOA 17 2. A correlation between temperature, ammonium concentrations and AOB and AOA growth during the composting process was observed by Oishi et al. 23. However, the relationship between environmental factors and AOB and AOA communities has not been well understood, especially in the composting process, which is of importance for the understanding of nitrifiers, nitrification, and control of nitrogen loss.
In this study, the community structure and diversity of AOB and AOA were investigated in the process of cattle manure composting. The relationship was explored between the community dynamics of AOB and AOA, and environmental factors (temperature, pH, water content, ammonia concentration, nitrate and total nitrogen and urease).
Materials and methods
Compost samples
Composting of rice straw and cow manure mixed in the ratio 5:1 (dry weight) was performed in an aerated vessel (50cm x 50cm x 110cm) for 29 days. Chemical and physical analyses of the composting mixture are listed in Table S1. The vessel was insulated to keep the heat produced during composting and air was pumped into it from the bottom of the vessel. The compost was turned manually on days 8, 15, 25. Samples were collected at different phases of composting and were named N1, N2, N3, N4, N5, N6 and N7. In detail, initial compost N1 (day 0), mesophilic stage N2 (day 1), thermophilic stage N3 and N4 (days 4, 7), cooling stage N5 and N6 (days 13, 23), and maturity stage N7 (day 29), stored at 4°C for physicochemical analysis and at -80 °C for DNA extraction.
Environmental factor determination
In this study, the environmental factors including temperature, pH, water content, total nitrogen (TN), ammonium concentration, nitrate concentration and urease activity were determined.
Compost temperature in the vessel was monitored every day by three thermometers inserted at different depths of the compost. Samples were dried at 105 °C for 24 h to constant weight to determine water content. pH was measured with a digital pH meter (Sartorius PB-10) in the compost suspension in the ratio 1:10 (w/v). To measure the contents of NO 3 -N and NH 4 +-N, 5g compost samples were extracted with 100 ml 2M KCl, and suspension was passed through Whatman filter paper No. 10. Nitrate was analyzed using an ultraviolet spectrophotometric method, while ammonium was analyzed by the indophenol blue method in a continuous flow analyzer. Total nitrogen (organic nitrogen and inorganic nitrogen) was measured using the Kjeldahl method 31. Urease activity was determined by the colorimetric method 32.
DNA extraction, PCR-DGGE and sequencing
DNA was extracted and purified using the E.Z.N.A. DNA Kit (OMEGA Inc, USA) and the DNA Purification Kit (Eur x, Poland), respectively, according to the manufacturer's instructions. Archeal amoA genes were amplified using primers Arch-amoAF and Arch-amoAR, while bacterial amoA genes were amplified using amoA-1F and amoA-2R primers (Table S2). A forty base pair GC-clamp, 5 -CGCCCGCCGCGCGCGGCGGGCGGGGCGGGGGCACGGGGGC-3', was attached to the 5' end of the forward primers 34. Polymerase chain reaction (PCR) and denaturing gradient gel electrophoresis techniques (DGGE) were performed according to the method proposed by Yamamoto et al. 37. The DGGE profiles were normalized and analyzed using Quantity One software (version 4.6.7; Bio-Rad, California, USA). DGGE bands were excised from the polyacrylamide gel and reamplified. The reamplified PCR products were then sequenced by Gene Company.
Phylogenetic analysis
The sequences of AOB and AOA were assembled and compared using BLAST from the DNA Databank. Phylogenetic analysis of ammonia-oxidizing communities was conducted using Molecular Evolutionary Genetics Analysis (MEGA) software version 5.0 with the neighbor-joining method 16. Clustering analyses were performed with DGGE bands using the UPGMA method. Pearson correlation analysis using SPSS 20.0 software was used to identify links between microbial diversity and environmental factors. Correlations between microbial community structure and environmental factors were also determined by the redundancy analysis (RDA) using CANOCO 4.5. The amoA gene sequences obtained in this study were available in the GenBank databases under accession numbers KR819833, KR818934, KR818937, KR818938 and KR818939 for AOA, and KT314092, KT314093, KT314094, KT314095, KT314096, KT314097, KT314098, KT314099, KT314100, KT314101, KT334410, KT334411and KT3344112 for AOB.
Results
Environmental factors
The dynamics of composting temperature was demonstrated in Figure 1A and based on the temperature, the composting process was divided into four stages: the mesophilic stage (days 0-3), the thermophilic stage (days 4-12), the cooling stage (days 13-23) and the maturity stage (days 24-29). On day 3 of composting, the temperature rose to 55 °C and was maintained above that level for 4 days, reaching the sanitary standard for manure treatment 13. The pH value of the compost samples varied between 8.010 and 8.800 ( Fig. 1B). Final pH (8.103) was in accordance with the standard (7.0-8.5) for mature compost 19. Water content declined from 67.0% at the beginning of composting to 32.3% at the end ( Fig. 1B). Figure 1C shows that at the beginning of the process, ammonia concentration increased, reaching the peak of 1400mg/kg on day 4, and then declined, often with a slight rise during the cooling stage and the maturity stage. Nitrate concentration increased gradually and reached the peak on day 4. The change of total nitrogen (ranging from 15.03 to 23.75g/kg) in the compost showed a "V" curve. During the composting process, urease activity increased in the early stage, reached maximum value (630 μg/g h) on day 4 ( Fig. 1D), and then declined until the end of composting.
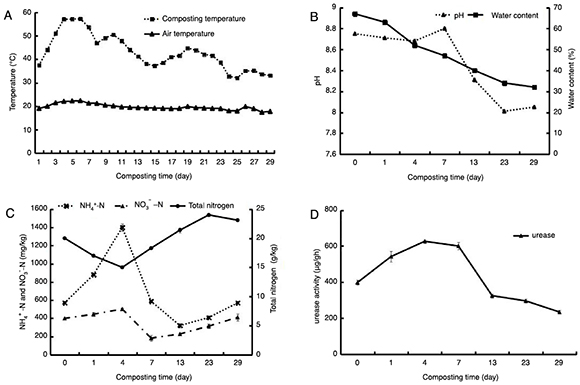
Figure 1 Changes of environmental factors during the process of composting. (Temperature change (A), pH and water content (B), concentration of total nitrogen, NH 4+-N and NO 3 -N (C) and urease activity (D). Samples: N1 from Initial compost (day 0), N2 from mesophilic stage (day 1), N3 and N4 from thermophilic stage (days 4, 7), N5 and N6 from cooling stage (days 13, 23), and N7 from maturity stage (day 29)).
Community structure of AOB
DGGE band patterns of AOB from different samples changed greatly ( Fig. 2A), with more bands observed in the mesophilic stage, the early thermophilic stage (day 4) and the cooling stage, fewer bands in initial compost (original composting materials) and the maturity stage and no bands in the late thermophilic stage (day 7).
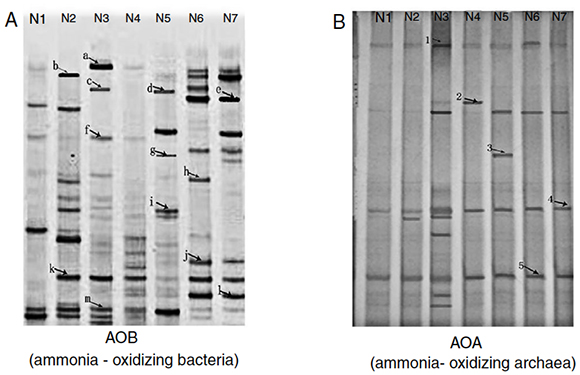
Figure 2 DGGE profiles of AOB (A) and AOA (B) communities in compost samples. (Samples: N1 from Initial compost (day 0), N2 from mesophilic stage (day 1), N3 and N4 from thermophilic stage (days 4, 7), N5 and N6 from cooling stage (days 13, 23), and N7 from maturity stage (day 29)).
Bands b and k, which were present in the mesophilic stage and disappeared in the thermophilic stage. This could be explained by the fact that the nitrifiers represented by these bands were unable to grow under thermophilic conditions. In addition, this change in AOB population might be also linked to a rapid increase in ammonia concentration in the compost. Bands a, c, f and m were present in the early thermophilic stage (day 4); however, no AOB were found in the sample taken in the late thermophilic stage (day7). This could probably be caused by the further increase in temperature. It was assumed that AOB could only survive high temperatures to some extent and excessively high temperature, for example more than 57 °C (day 5), might not be favorable for AOB growth.
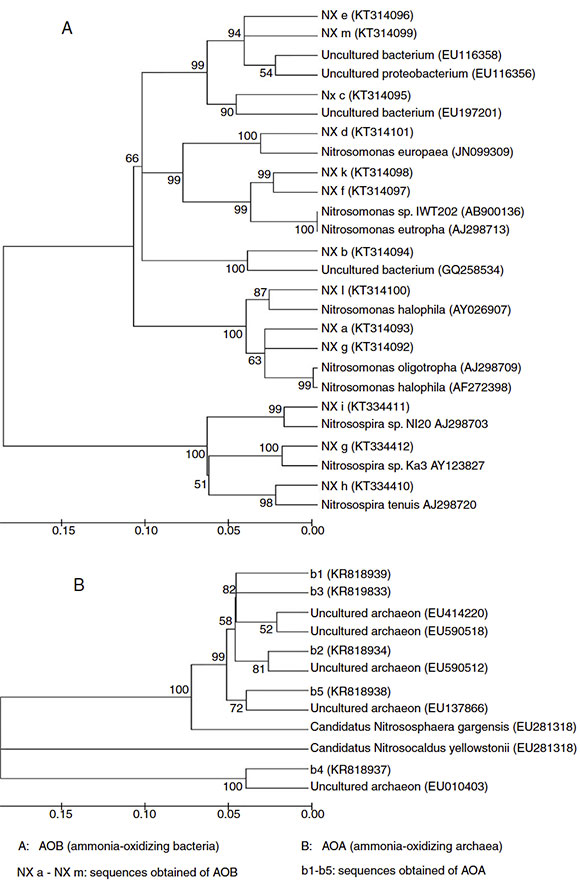
A total of 13 AOB sequences were obtained from the DGGE bands by sequencing. The sequences represented by bands a, d, f, g, k and l, which were distributed at different stages during composting, were identical to an amoA gene sequence obtained from Nitrosomonas spp., indicating that this genus was predominant during the composting of cattle manure. The nitrifier represented by band f was identical to the amoA gene sequence obtained from a Nitrosomonas eutropha (AJ298713) ( Fig. 3A), which was usually detected in environments with high-ammonium and pH 7 10. These results were consistent with our finding that band f presenting Nitrosomonas eutropha was observed in the compost of the early thermophilic stage (day 4), where high ammonium concentration and pH were detected. Nitrifiers represented by bands h, i and g were closely related to Nitrosospira spp., and bands b, c, e and m were identical to the uncultured bacterium.
Community structure of AOA
As shown in Figure 2B, the DGGE patterns of AOA were different from those of AOB. Two dominant DGGE bands (band 4 and 5) were observed in all compost samples and more bands were observed in the compost sample taken from the early thermophilic stage (day 4) than from the other stages, indicating that the condition in this stage might be more favorable for AOA growth.
A total of 5 AOA sequences were obtained from the compost samples. AOA sequences represented by bands 1 and 2 were from samples taken in the thermophilic stage, bands 3 and 5 in the cooling stage and band 4 in the maturity stage. However, no AOA sequence was detected using the primers employed in the precomposting stage and in the mesophilic stage, although DGGE bands were observed in the two stages, indicating that the DGGE bands obtained did not necessarily represent AOA sequences. A phylogenetic tree was constructed based on the AOA sequences ( Fig. 3B). The sequences of bands 2 and 3 were closely related to the amoA gene carried by the uncultured archaeon (EU590512).
Diversity of AOB and AOA communities
Richness (S) values were calculated as the number of DNA bands detected in the respective lane of the DGGE profile, while the Shannon-Wiener index (H) and evenness (E H) values were calculated based on the equations H = 1J2P> (ln pi) and E h = H/H max = H/ln S, respectively, where p <sub>t was the ratio between the intensity of the specific band and the total intensity of all bands and S was the total number of bands in each sample.
Richness and diversity of AOB and AOA amoA genes in different stages of the composting process was shown in Table 1. Bands of AOB and AOA included 7-14 and 6-15, respectively. Community diversity of AOB and AOA decreased in the early thermophilic stage (day 4), and the lowest Shannon-Wiener index of AOB and AOA was observed in the early thermophilic stage (N3 sample), increasing in the late thermophilic stage and the cooling stage. The community diversity of AOA (Shannon-Wiener index = 1.936-2.383) was higher than that of AOB (Shannon-Wiener index = 2.072-2.321) during the composting process.
Comparison of AOB and AOA communities
The differences between AOB and AOA communities from different compost samples were compared using the phylogeny-based UPGMA clustering analysis. As shown by the data in Figure S1A, sample N1 was clustered together with samples N2, N4 and N5 with the low distance value 0.28, and separated from samples N3, N6 and N7, which indicated that AOB communities obviously changed with the composting process. Sample N3 was distantly separated from the other samples, which revealed that changes in AOB communities were more drastic in the thermophilic stage than in the other stages. Samples N6 and N7 samples were also well clustered together (with the distance value 0.44). The results showed that changes in AOB communities tended to be stable in the cooling and maturity stages. Sample N1 separated from the other samples, which indicated that there was an obvious change in AOB communities with the composting process. The results above indicated that the samples collected from the same stage could have a highly variable AOB community structure, while the samples at different stages could have a similar AOB structure.
With respect to AOA, Figure S1B shows that samples N1, N5 and N7 were grouped together, and so were samples N6 and N3, and samples N4 and N2. However, changes in the distance values were not obvious. The results suggested that the different stages of the compost samples might have a similar AOA community structure, or that there were lower contents of the AOA community in the cow-dung compost.
Correlation between environmental factors and ammonia-oxidizer community structure
The correlation between the AOB and AOA community structure and environmental factors was illustrated using a redundancy analysis (RDA) approach. The arrowed vector lines in this work represent the environmental factors. The correlation between the AOA community structure and environmental factors was reflected by either the angle between the line and the axis or the length of the line, the smaller angle or the longer line, the higher correlation.
AOB
The environmental factors in the first two RDA axes, explained 45.5% and 34.4% of the total variance in AOB community structure ( Fig. 4A). As indicated in Figure 4A, pH, total nitrogen (TN) concentration, nitrate concentration and water content were positively correlated with the AOB community structure while ammonium concentration was negatively correlated with it. The fact that the arrowed vector lines of TN and water content were longer than those of other environmental factors indicated that water content B: AOA (ammonia-oxidizing archaea) and TN were more relevant to the AOB community structure, revealing that there was a high correlation between ammonium concentration and the AOB community structure based on the finding that the angle between the arrowed vector line of ammonium concentration and the axis was the smallest compared to other factors.
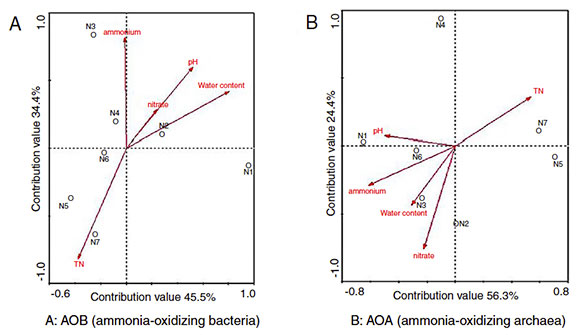
Figure 4 RDA ordination plot showing the relationship between the environmental factors and ammonia oxidizer community structure. (Environmental factors: pH, water content, total nitrogen (TN), ammonium and nitrate concentrations).
Based on the RDA, the distance between two samples could reflect the difference of the ammonia-oxidizing bacteria community structure, the shorter distance, the smaller difference and vice versa. As demonstrated in Figure 4A, longer distances were observed between samples (N1, N2 and N3) in the mesophilic stage and the early thermophilic stage, indicating the dramatic succession of the AOB community observed in these stages. On the other hand, the shorter distances between samples N4, N5, N6 and N7 suggested that changes in community structure were less obvious in the cooling stage and the maturity stage of composting.
Water provides a place for dissolution and transport of nutrients required for physiological activities of the microorganisms in the compost. In the range of 30-60% water content, higher water content allows higher microbial activities 18. During the composting process in this study, water content declined from 67.0% to 32.3% in the composting process ( Fig. 1B); therefore, variation of water content might have an impact on the AOB community structure.
AOA
Environmental factors such as pH, water content, temperature, dissolved oxygen, nitrogen, and organic matter were relevant to the distribution of AOA 6. In this study, environmental factors in the first two RDA axes explained 56.3% and 24.4% of the total variance in the AOA community structure ( Fig. 4B). TN, water content, ammonium and nitrate concentrations were positively correlated whereas pH was negatively correlated with the AOA community structure. Furthermore, higher correlations between the AOA community structure and concentration of ammonia, nitrate and TN were found based on the arrowed vector lines.
Figure 4B demonstrated the longer distances between samples N4, N5, N6 and N7, and the shorter distances between samples N1, N2, and N3. The results indicated that the community succession of AOA was more dramatic in the late thermophilic stage, the cooling stage and the maturity stage, whereas the community changes were less obvious in the initial compost and the mesophilic stage.
Correlation between environmental factors and AOB and AOA community diversities
During the composting process, correlations between the diversities of AOB and AOA and environmental factors were shown in Table 2. AOB community diversity was negatively correlated with pH (r = -0.938, p < 0.01) and water content (r = -0.765, p < 0.05), and positively correlated with TN (r = 0.894, p < 0.01). The content of TN in the compost is much higher than that in soils; the difference in TN magnitude might contribute differently to the AOB community diversity.
Discussion
AOA and AOB structure variations during composting
The sequence of band 1, which occurred in the early thermophilic stage, was similar to that in the uncultured archaeon (EU414220), which was also found in the high temperature environment (hot spring) reported by Hatzenpichler et al. 8. This result suggested that the AOA represented by band 1 was probably thermophilic. Sequences of bands 4 and 5, which were observed in the maturity stage and the cooling stage of composting respectively, were very similar to that of the amoA gene carried by the Candidatus Nitrososphaera gargensis (EU281318). However, Oishi et al. 23 found that Candidatus Nitrososphaera gargensis was the predominant AOA in the thermophilic stage. Zhalnina et al. 38 demonstrated that with the change in ammonium concentrations, the community structure of AOA also changed and at different ammonium concentrations (high, medium and low) different species of AOA were detected. It was reported that the high concentration of ammonium had the ability to inhibit the assimilation of CO 2 in thermophilic archaea, indicating that a high level of ammonium could inhibit ammonia oxidation and thereby exert influence on the community structure of AOA 8. These results with regard to the Shannon-Wiener indices in Table 1 were in accordance with the reports of other researchers who found that AOA usually had relatively higher community diversity than AOB in soil ecosystems 21 7 8 34 36.
An excessively high ammonium concentration might be also responsible for the inhibition of nitrifier growth in the thermophilic stage. This assumption was supported by the research conducted by Oishi et al., who found that AOB hardly grew when the temperature exceeded 60 °C and at high ammonia concentration 23. Bands d, g, h, i and l were observed in the cooling stage and band e in the maturity stage. Di et al. found that AOB could grow in a high ammonium concentration soil, while low ammonium concentration was suitable for AOA growth. In this study, ammonium concentration changed dramatically during composting, which might contribute to the variation in AOA community 3.
Correlation between environmental factors and AOA and AOB structure
Water content was related to the concentration of dissolved oxygen, which was a crucial factor for the growth of aerobic microorganisms and thereby might also exert some influence on microbial activities. A previous study showed that when the dissolved oxygen concentration was lower than 1.0 mg/l, the ammonia oxidation process might involve both AOB and AOA. However, when dissolved oxygen concentration was higher than 2.0 mg/l, AOB might be the main participant in the process of ammonia oxidation 33. AOB are aerobic microorganisms and their growth might be influenced by the decline in water content during composting, which could probably contribute to the variation of dissolved oxygen.
Nitrification is the microbial oxidation of ammonia to nitrate, therefore as the substrate of the nitrifier, ammonia played an essential role in the growth of AOB 5. Ammonia might become a limiting factor for the growth of AOB when its concentration was low in the environment 30. However, a high level of ammonia might have a negative impact on microbial activities including AOB. Furthermore, a high concentration of ammonia led to a higher pH value, which might also inhibit AOB growth subsequently. In the present study, ammonia concentration changed dramatically during composting with the maximum level observed in the early thermophilic stage (day 4). This variation of ammonia concentration could probably contribute to the shift in the AOB community, which changed greatly during composting. In the late thermophilic stage (day 7) of composting, no AOB sequences were detected in this study, the explanation being that the maximum ammonia concentration in the early thermophilic stage (day 4) might have exerted delayed inhibiting impact on AOB growth although the high temperature might also have contributed.
A higher correlation was found between the AOA community and nitrate concentration in this research, which was consistent with Zhang et al.'s results 39. As the end product of the nitrification process, nitrate might have feedback effects on the ammonia oxidation process, which is the starting step of the reaction, and thereby inhibits AOA community growth 38. On the other hand, the variation in the AOA community, which provides substrate for the production of nitrate, could influence the concentration of nitrate nitrogen. Therefore, it was explainable that a higher correlation exists between the AOA community and nitrate concentration during composting.
Total nitrogen generally consists of four components, namely ammonia, nitrate, nitrite and organic nitrogen. As the intermediate species between ammonia and nitrate, nitrite was therefore closely related to ammonia and nitrate. Organic nitrogen was the source of ammonia through ammonification, and hence, it was also closely linked to ammonia. In this study, both ammonia nitrogen and nitrate nitrogen were highly correlated with the AOA community in the composting process. As a result, it was reasonable to conclude that TN was highly correlated to the AOA community. In addition, alkenes and various aromatic hydrocarbons in organic nitrogen could inhibit the oxidation of ammonia monooxygenase to ammonia and could exert great impact on the activity of nitrifiers (AOB and AOA) 14.
Diversity of the AOB community positively correlated with TN (r = 0.894, p < 0.01). However, Dai et al. 2 reported that AOB diversity was negatively correlated with TN in soil. AOA community diversity was negatively correlated with ammonium concentration (r = -0.901, p < 0.01). This result was consistent with Ding et al. 4 who suggested that the diversity of the AOA community in plateau soil might be affected by TN.
Conclusion
In this study, most AOB were closely related to Nitrosomonas spp., Nitrosomonas eutropha, and Nitrosospira spp. Most AOA sequences belonged to Candidatus Nitrososphaera gar-gensis. During the composting process, the AOA community showed a higher Shannon-Wiener index than that of the AOB community. pH and water content variation were negatively correlated with AOB community diversity, while positively correlated with TN variation. AOA community diversity was negatively correlated with NH 4+-N. In addition, NH 4+-N showed a great impact on the succession of AOB and AOA communities during composting.












 uBio
uBio