Introduction
Herbicides based on glyphosate (HBGs) can be applied sev-eral times throughout the year, even when the crops are growing or between growing seasons in order to hasten the drying of weeds30. In Argentina this agricultural system has led to the development of GP-resistant weeds, causing an increase in both GP doses and applications. Therefore, it was necessary to apply different formulations of herbicides to achieve the control of all weeds. The Humid Pampa region of Argentina is responsible for 80% of soybean production with intensive use of agrochemicals, HBGs being the most applied herbicides35.
Recent studies have revealed that N-phosphonomethylglycine (GP) and its main degradation metabolite aminomethylphosphonic acid (AMPA) may persist in soil for long periods8. The residues are found at all levels of the food chain, such as drinking water, plants, animals, and even in exposed humans34. The presence of GP and AMPA has been reported in water and sediments of streams from rural and suburban basins of our country within the provinces of Buenos Aires, Santa Fe and Córdoba2,41,43,18. The extensive use of this herbicide has shown to cause a selection in soil microbial populations, with increases in populations of specific microbial taxa with capacity to degrade the herbicide31.
The reports of International organizations such as the Food and Agricultural Organization of the United Nations have alerted about pesticide pollution21. In this context, several researchers worldwide have focused their atten-tion on the development of strategies for minimizing the pesticide contamination of natural resources and remediating contaminated natural environments37. The bioaugmentation of contaminated sites with degrading microorganisms is one of the most promising bioremediation strategies. The soil is the main receiver of pesticides used in agriculture. After the application of HBG, a part of the herbicide is adsorbed or immobilized to the soil particles and the rest can leach into other ecosystems. The permanence of GP not linked to the soil depends on the photo, chemical, and microbial degradation rate. Bacteria and fungi are considered to be the most important microorganisms involved in biotic pesticide degradation. Mycelial growth and extracellular enzymes provide filamentous fungi an advantage over other microorganisms such as bacteria and yeasts. Furthermore, in acidic medium, soil fungal degradation prevails in the proximity of the surface where the oxygen and the organic matter is high6. In this context, fun-gal enzymes are important in biotechnology processes and are promising in organophosphorus herbicide degradation. These processes include mineralization, co-metabolism and interspecific coordination metabolism37. Several reports have reported the ability of native soil microfungi such as Aspergillius, Penicillium and Fusarium in organophos-phorus pesticide degradation6. In previous studies Carranza et al.15,12,13,16 showed that non-toxigenic Aspergillus section Flavi strains isolated from soil exposed to pesticides were able to tolerate in vitro high doses of GP (500 mM), and were successfully able to use the herbicide as both phosphorus or nitrogen source. The degradation percentages were higher than 50% at 15 days of incubation. These strains were tested on soil microcosms showing GP tolerance, permanence and
Mycobiota from agricultural soils and their tolerance to glyphosate
competitiveness in the presence of native mycota. Considering that the contaminated sites are the major source of degrading microorganisms and degradation of pesticides is not performed by a single microbial population6,37, the aims of the present study were to isolate the GP-tolerant cultur-able mycota in two soils with different pesticide exposure, from Córdoba, Argentina, and to evaluate the growth parameters in native fungal isolates in the presence of GP and the effective dose that caused 50% of growth reduction.
Materials and methods
Sampling sites and sample processing
Four fields were selected for soil sampling in the south of the Province of Córdoba. Three fields were chosen with a long history of exposure to pesticides (approximately 10 years). From each field, three samplings (10 samples were taken in each sampling) along the year were performed between 2016 and 2017. The fields were located in: place 1 - Coronel Moldes (34°28'18" 63°32'18"), place 2 - Serrano (33°37'00" 64°30'23") and place 3 - Espinillo (33°00'47" 64°19'13"). Additionally, representative soil samples from fields with-out direct exposure to pesticides, no-tillage and with native forests located in Córdoba, Argentina (place 4: 31°56'16" 64°37'11") were analyzed. Twenty samples were collected from them in 2017.
Soil samples of 1 kg were collected from the surface layer (at a depth of 10cm) of the soil. These samples were homogenized and airdried for 1 -2 days at 25-30°C. Samples weighing 100g were thoroughly mixed and passed through a testing sieve (2 mm mesh size) to separate the soil from the debris. Samples were stored at 4 °C and the isolation of fungi was performed within 2 days after the sampling.
Glyphosate solutions
A commercial formulation (Roundup Controlmax®, Buenos Aires, Argentina) was used in this study. A stock herbicide solution of 2 M of the active ingredient was prepared dissolving the appropriate quantity of commercial product in sterile distilled water (100 ml). The herbicide solution was sterilized (filters of 0.2 ^m, Microclar, Buenos Aires, Argentina) and maintained at 4°C until use.
Enrichment and isolation of fungi in media supplied with GP as carbon, nitrogen and phosphorous sourcesTen grams of each soil sample were added in Erlenmeyer flasks containing 50 ml of sterile modified Czapeck-Dox medium (CZD), with the following composition (l); 10g glu-cose, 0.5g MgSO4-7H2O, 0.5g KCl, 2g NaNO3, 0.01 g FeSO4, 1g KH2PO4, 0.5g yeast extract, gentamicin and chloram-phenicol (100mg/l each) to inhibit bacterial growth. CZD without glucose, NaNO3 or KH2PO4; and supplied with GP at a final concentration of 10 mM (CZDC); 1.5 mM (CZDN) and 1.0 mM (CZDP), respectively were prepared to evalu-ate the use of GP as sole source of carbon, nitrogen and phosphorus33. The CZD media were autoclaved at 121 °C for 15 min and the appropriate aliquot of GP was added to the sterilized culture media template at 45-50°C to obtain the final concentrations mentioned above. These concentrations were selected based on the percentages that the carbon, nitrogen or phosphorus sources should be present in the fungal media. Each condition (CZDG, CZDN and CZDP) was evaluated by duplicate. Then, the flasks were incubated at 28 °C on a shaking platform at 120 rpm for 7 days. After the incubation period, serial dilutions from 10-1 to 10-6 were performed on 0.1% peptone water solution and then aliquots of 0.1 ml were inoculated by the surface-spray method by duplicate on Petri plates containing CZDC, CZDN and CZDP media with 1.5% agaragar. The plates were incubated in the dark at 28 °C for 7 days. After the incubation period, the plates that contained 10-100 colonies were used for total and genera counting.
Morphological identification at the genus level of the different colonies was conducted by sub-culturing each colony on Malt Extract Agar (MEA). The identification was performed through macroscopic and microscopic criteria following taxonomic keys42,46,49. The results were expressed as colony-forming units (CFU) per gram of soil of the total mycota and each genus in each medium supplied with GP. The strains were incorporated into the culture collection of the Department of Microbiology and Immunology, National University of Río Cuarto, Córdoba, Argentina, and are maintained in 15% glycerol (Sigma Aldrich, St. Louis, MO, USA).
In vitro screening of GP tolerance of fungal isolates at optimal water availability and temperature conditionsAll the strains that were isolated from the soils either exposed or not exposed to pesticides were subjected to three successive cultures in MEA supplied with 10 mM of GP for screening their capacity to grow in the presence of the herbicide. Of these, eighteen fungal isolates belonging to Aspergillus, Trichoderma, Mucor, Fusarium and Penicillium genera were selected to evaluate GP tolerance. All strains were isolated from exposed soils, except for Trichoderma spp. (strain 311). Lag phase prior to growth, growth rate and the effective dose that caused 50% of growth rate reduction (EC50) in the presence of GP were evaluated in order to estimate GP tolerance.
The CZD medium supplied with the solution of GP at final concentrations of 5, 20, 50 and 100 mM (equivalent to 0.85, 3.4, 8.4, and 16.9mg/ml, respectively) was used for this assay. The lowest GP concentrations tested are the field application rates recommended and the highest ones represent the concentrations of pesticide reported in sites with spill38. The water activity (aW) of the medium was conditioned at 0.98 with glycerol to simulate the optimal conditions of growth. The aW of the medium was checked with AquaLab Series 3 (Decagon Devices, Inc., Pullman, WA, USA). In addition, the respective controls without GP were prepared. The culture plates for each treatment were needle-inoculated centrally with a spore suspension (106 spores/ml). The inoculum was prepared from 5- or 7-day-old cultures on MEA medium. Each treatment was done in triplicate and incubated at 25 °C for 15 days or until the fungal colony reached the edge of the plate. All the experiment was repeated twice.
Growth rate was calculated taking two measures of colony diameter at right angles to one another daily from each replicate plate. The radius of the colony was plotted against time, and a linear regression was applied in order to obtain the growth rate as the slope of the line to the X-axis. The lag phase (h) before exponential growth was also determined7. The percentage of growth inhibition by GP was calculated for each isolate in each treatment in order to determine the EC5040.
Statistical analysis
Data from the fungal count, growth rate and lag phases were subjected to analysis of variance. Means were compared using a linear mixed model and the Fisher’s protected least significant difference (LSD) test to determine the significant differences between the means from total fungal counts and frequency of fungal genera. All data were transformed to log10(x +1) to obtain homogeneity of variances. Means were also compared using the Fisher’s protected LSD test to determine the influence of concentration of herbicide on the growth rate and lag phase prior to growth of the tested strains. The statistical analysis was performed using InfoStat Professional software version 201 720.
Results
Enrichment and isolation of fungi in media supplied with GP as carbón, nitrogen and phosphorous sources
Figure 1 shows the mean values of total culturable mycota counts (log10CFU/g) from different sampling places in control media and supplied with GP. Total fungal counts varied according to sampling place. Counts ranged between 5.5 and 8.7 log10 CFU/g. The highest values were observed in soil samples without pesticide exposure or no-tillage (place 4) (p <0.05). In this place the highest counts were found in control media, followed by CDZN, CZDP and CDZC media. While in exposed places, the highest counts were found in media supplied with GP, and the counts varied according to the media supplied with GP analyzed.
With regard to the analysis of the culturable mycota, 13 genera of filamentous fungi were isolated. Fusarium spp., Aspergillus spp., Mucor spp., Penicillium spp., Tri-choderma spp., Paecilomyces spp., Cladosporium spp., Verticillium spp., Exophiala spp., Alternaria spp., Phoma spp., Phialophora spp. and Streptothrix spp. were isolated in all soil samples. Some genera, such as Alternaria spp. and Streptotrhix spp. were found only in non-exposed soil samples, with mean counts of 7 and 6log10CFU/g, respec-tively. The genera Trichoderma spp., Paecilomyces spp. and Verticillium spp. were found only in the soils exposed to pesticides, with mean counts of 6.4, 5.4 and 6.3 log10 CFU/g, respectively (data not shown).
Figure 2 shows the counts of prevalent genera from different places in control and GP-supplied media as sole source of nitrogen, phosphorous and carbon. The genera Fusarium spp., Aspergillus spp., Mucor spp., Penicillium spp. and Sterilia spp. were the prevalent fungi isolated from soils both exposed and not-exposed to pesticides. They were isolated in all the media tested, except Mucor spp., which were only isolated in control and CZDP medium without significant differences between both media. A different behavior was observed in the other genera. Fusarium spp. showed similar counts in control and CZDN and CZDP media. For Aspergillus spp. the highest count was found in the CZDP medium. While for Penicillium spp. this behavior was observed in CZDP and CZDN. For Sterilia spp. no significant differences in the count were found among the supplied GP media (p <0.05).
In vitro screening of GP tolerance of fungal isolates at optimal water availability and temperature conditionsTable 1 shows the lag phase prior to growth and EC50 of 18 isolates in media with increasing GP concentrations at optimal water availability and temperature conditions. Lag phase varied depending on the isolate tested. In general, the presence of GP in the media produced a time of adaptation similar or higher than those in the control media. For most of the isolates, the highest GP concentration (100 mM) produced longer lag phases. Only one isolate (Penicillium spp., 139) did not develop in this condition (the lag phase was extended more than the total incubation time, 360 h). Contrarily, Mucor spp. iso-lates showed the lowest values of this parameter with 100 mM of GP, especially isolate 182 remained only 4h in the adaptation phase. For Aspergillus spp. and Sterilia spp., no significant differences in the length of the lag phase prior to growth with the increase of GP concen-tration was observed with respect to control. However, for isolates 128 (Aspergillus spp.), 133 and 80 (Sterilia spp.), the longest lag phases were also observed with 100mM of GP (p<0.05). For Trichoderma spp. the behavior depended on the isolate tested. No significant differences between the lag phases in media supplied with GP and controls were observed for isolate 311, while for isolate 140, the longest lag phase was observed with 5mM of GP. Likewise, for Trichoderma spp, for Penicillium spp. this growth parameter varied according to the isolates tested. In general, the longest lag phases were observed at the high-est GP concentrations, and similar values were observed between controls and 5-50 mM media supplied with GP (p <0.05).
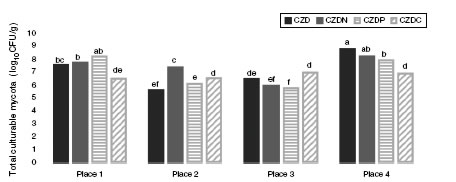
Figure 1: Mean values of total fungal counts (log10 CFU/g) from the different places sampled in control media (CZD) and supplied with GP as only source of nitrogen (CZDN), phosphorus (CZDP) and carbon (CZDC). a,fMean values with different letters indicate significant differences in accordance with the Fisher’s LSD test (p <0.05).
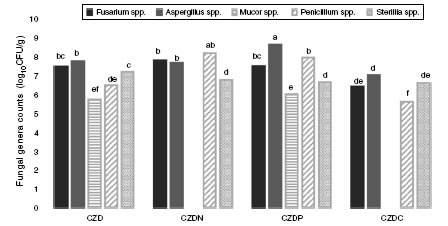
Figure 2: Mean values (log10CFU/g) of the prevalent genera from different places in control media and supplied with GP as only source of nitrogen (CZDN), phosphorus (CZDP) and carbon (CZDC). a,eMean values with different letters indicate significant differences in accordance with the Fisher’s LSD test (p<0.05).

Table 1: Effect of different concentrations of GP on the lag phase (h) and EC50 of different isolates in CZD medium at 0.98 aw and 25 °C.
With respect to EC50, the lowest value (<5mM) was observed for Penicillium spp. While the highest value (>100 mM) was observed for Trichoderma isolates, followed by Sterilia spp. (isolates 133 and 135) with values of 100 mM, two Aspergillus spp. and one Mucor spp. (isolate 166) that had an EC50 value between 50 and 100 mM. In addition, EC50 values of 50 mM were found in Sterilia sp. (isolate 78) and Mucor spp. (isolates 182 and 208) and EC50 values of 20-50mM in Sterilia spp. (isolate 80).
Figure 3 shows the growth rate of the 18 isolates in control media and in the presence of different GP concen-trations at optimal water availability and temperature. The isolates developed with a different behavior in all GP con-centrations assayed. The growth rates for Sterilia spp. and Aspergillus spp. decreased as GP increased, this reduction being more significant at the highest concentrations (Fig. 3A and D) (p < 0.05). For Trichoderma spp. the growth rate was constant with the increase of GP, e.g. isolate 140 showed a significant increase in growth rate with respect to the control with 50mM (Fig. 3B). For all Mucor isolates, similar growth rate values were observed between the control media and the media supplied with 5 and 20 mM of GP. Furthermore, significant reductions of this parameter were found with 50mM of GP (Fig. 3C). In general, for Penicillium spp., a decrease in growth rate was observed with the increase in GP concentration, being more evident with 50 mM. Only one isolate (139) did not develop at the highest herbicide concentration, while, with 5 and 20 mM of GP, similar or higher growth values were observed compared to the corresponding control (Fig. 3E) (p <0.05). The isolates that showed the highest growth rates in media supplied with dif-ferent GP concentrations were isolate 2 (Aspergillus spp.), isolates 182, 166 and 208 (Mucor spp.) and isolates 140 and 311 (Trichoderma spp.)
Discussion
The culturable mycota assay showed that the filamentous fungal count from the fields of Córdoba province varied according to the origin of the samples (exposure or non-exposure to pesticides) and the isolation media analyzed. Noticeable higher counts of culturable mycota in no-tillage or non-exposed soils to pesticides were observed compared to those in tillage and exposed soils both in controls as in the media supplied with GP. These results are partially in agreement with Cabello and Arambarri10 who evaluated the rhizospheric soil microfungi from a native forest (undis-turbed and disturbed) from Buenos Aires, Argentina. They found highest counts in undisturbed soils. These authors informed that in these latter soils the number of colonies was 2-fold greater than the number found in disturbed forest at the same time. These results partially agree with the present study as the counts in non-exposed soils were higher than those in exposed soils; however, the differences were not as marked. The saprophytic mycota plays an important role in the cycles or nutrients in the ecosystems, because their capacity of fragmentation of organic polymeric matter allows the last mineralization by other microorganisms19. The high percentage of organic matter reported in soils without human intervention1 can explain the high counts observed in the non-exposed agricultural soil sampled in this work.
The most prevalent genera were Fusarium spp., Aspergillus spp., Mucor spp. and Penicillium spp., both in exposed and non-exposed soils. While Trichoderma spp., Paecilomyces spp. and Verticillium spp. were found only in exposed soils. These results agree partially with Carranza et al.15 and Nesci et al.39, who informed a high frequency of Aspergillus spp., Penicillium spp., Fusarium spp., Cladosporium spp., Trichoderma spp. and Paecilomyces spp. isolated from maize fields in the south of Córdoba province exposed to pesticides and tillage practice. Only Trichoderma spp. and Paecilomyces spp. were the genera isolated only from non-exposed soils in the present study.
The main fungal genera isolated in this work were similar to a general isolation pattern found in non-rhizospheric soil ecosystems5 and in soils exposed to GP45,36. The isolation of fungi such as Penicillium, Aspergillus, Trichoderma and Cla-dosporium is very common mainly in soil and organic plant material since the soil is the primary ecosystem for these fungi. They produce different propagation and resistance structures that provide them with adaptive features for environmental unfavorable conditions42 that explain their capacity for the fast invasion of the available substrate23. These characteristics favor the maintenance of the species in the ecosystem. Wardle and Parkinson48 and Reis Valpassos et al.44 suggested that pesticides could change the sapro-phytic capacity of soil fungi or produce selective pressure that affects microbial activity favoring the development of more adapted genera. The presence of the same prevalent genera in exposed and non-exposed soils suggested that they possess major survival capacity to disturbers such as pesti-cide contamination and tillage.
Prevalent fungal genera counts in media supplied with GP showed differences according to the media analyzed. Ster-ilia spp. showed good development in all media, while Mucor spp. could only develop in media with GP as phosphorous source. The genus Fusarium spp. and Penicillium spp. developed in media with GP as nitrogen and phosphorous source, and Aspergillus spp. showed remarkable development with GP as phosphorous source. These results are indicating that the herbicide is used as a nutrient and the metabolic path-ways vary according to the genus. The fungal enzymatic capacity and the rapid growth allow a fast invasion of the substrate to be used as a nutrient27.
The length of the lag phase prior to growth and mycelial growth rate are the parameters used to indicate the effective development of the different isolates in the presence of GP at optimal aW and temperature. The lag phase varied according to the isolate. The increase of GP concentration in the media produced an increase in the adaptation time in all isolates. However, this behavior was not observed in isolate 405 (Mucor spp.), as the lag phase values diminished signi-ficantly (80%) with 100mM of GP with respect to control. These results agree with Barberis et al.7 who observed the same tendency in this parameter. They reported an increase in the lag phase at increasing GP concentrations (1-10mM) in toxigenic A spergillus flavus strains growing on maize-based medium.
The evaluation of this growth parameter suggests that the isolates needed in average an adaptation time higher than 30h (Aspergillus spp., Sterilia spp., Trichoderma spp.
and Mucor spp.) and 24.7h (Penicillium spp.) to start the exponential growth in media supplied with GP both at low and high concentrations. Although in the literature there are some studies regarding the impact of GP on fungal growth24,50,29, there is scant information about the lag phase in relation to herbicides. In previous works, Carranza et al.15 reported lower lag phase values (18-23 h) than in the present study in non-toxigenic Aspergillus sec-tion Flavi strains grown on low nutrient status media (soil based medium) conditioned at -0.70 of water potential and supplied with 5, 10 and 20 mM of GP. Contrarily for Aspergillus section Nigri strains, the lag phase values were higher (30-38 h) with 5, 10 and 20mM of GP also in soil-based medium conditioned at -0.70 of water potential14. In other study, an increase in the lag phase at increasing GP concentrations (100-500 mM) was reported for Aspergillus section Flavi strains grown on milled soybean extract agar12. For Fusarium verticillioides, Fusarium oxysporum and Fusar-ium graminearum higher lag phases (36-90 h) were also reported in maize meal agar conditioned at 0.98 of aW and supplied with 20, 30 and 50 mM of GP11. Despite the fact that the evaluated isolates came from pesticide-exposed environments, they needed an acclimation period for their development in media with GP as a nutrient source. In this period, the hydrolytic enzymes are induced to metabolize the organophosphorus compounds. In the present study, the isolates showed variability in lag phase duration according to the GP concentration assayed, only one strain did not develop at the highest concentration evaluated.
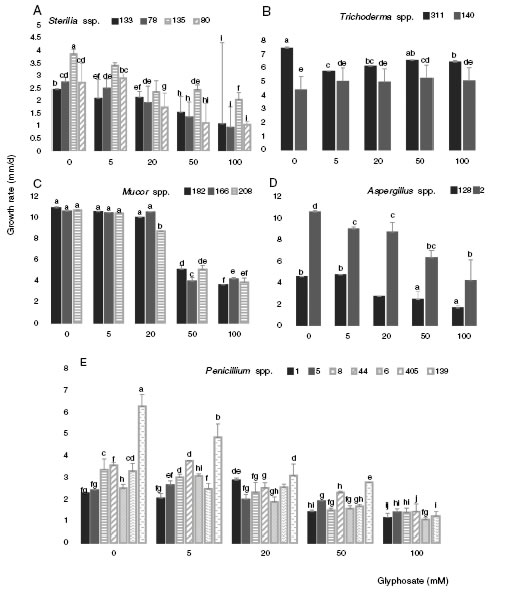
Figure 3: Growth rate of Sterilia spp. (133, 78, 135 and 80) (A), Trichoderma spp. (311 and 140) (B), Mucor spp. (182, 166 and 208) (C), Aspergillus spp. (128 and 2) (D), Penicillium spp. (1, 5, 8, 6, 44, 139 and 405) (E) in CZD media. a,i Mean values of growth rate with the same letter from each fungal genera are not statistically different according to the Fisher’s LSD test (p<0.05).
Fungi are, in general, more tolerant to high xenobiotic concentration present in polluting sites than bacteria22. Results of EC50 showed that all isolates, except Penicillium spp., were tolerant to GP. The Trichoderma isolates were the most tolerant (values >100 mM), followed by Sterilia spp. isolates 133 and 135 (values of 100mM), two Aspergillus spp. and Mucor spp. isolate 166 with values between 50 and 100 mM. There is not information about this parameter for the estimation of sensitivity of fungal strains to GP. Previously, Nwachukwu and Osuji40 reported tolerance to the herbicides atrazine, heptachlor and metolachlor of indigenous white rot fungus in soil extract agar. These fungi showed EC50 values of 0.011, 0.0038 mg/l and no inhibition or higher than 0.02 mg/l for atrazine, heptachlor and metolachlor, respectively. These EC50 values are notably lower than the ones found for Trichoderma, Mucor, Aspergillus and Sterilia isolates.
The growth rate varied according to the isolates tested. All of them could develop at all the GP concentrations assayed, except one Penicillium isolate (139) at the high-est GP concentration. For Penicillium spp., Sterilia spp., and Aspergillus spp., the growth rates decreased with the increase of GP. This behavior was previously reported by Carranza et al.12 with Aspergillus oryzae and non-toxigenic A. flavus growing on milled soybean extract agar and supplied with higher concentrations of GP (100-500mM). Contrar-ily, in other study, Carranza et al.15 reported a significant increase in the growth rate of A. flavus strains as the concentration of GP increased from 5 to 20 mM in soil extract solid medium conditioned at optimal water availability. Similar results were also found by Barberis et al.7 with toxi-genic Aspergillus section Flavi strains grown in maize-based medium and supplied with 5 and 10 mM of GP. In the same way, a positive correlation between the GP degradation rate and the biomass of A. oryzae AF-02 was informed by XueHua et al.50.
With respect to other genera, Krzysko-Lupicka and Orlik32 showed similar results with Mucor, Penicillium and Trichoderma strains isolated from soils. They grew exceptionally well in synthetic media supplied with GP as the sole source of phosphorus after a significant lag phase. While Klimek et al.29 reported opposite results with a strain of Penicillium crysogenum as the concentrations of 5, 10 and 25 mM of GP in CZD medium (the herbicide replaced the nitrogen source) stimulated the fungal growth rate. Similar results were informed by Bujacz et al.9 who reported that this herbicide inhibited the Penicillium notatum (now P. crysogenum) growth in CZD medium with the highest GP concentrations, while with 1-0.5mM the fungal growth sig-nificantly increased only in the media where the herbicide replaced the phosphorus source. With respect to Mucor spp., only one study performed in situ reported the effect of GP on this genus. Mandl et al.36 reported the absence of Mucor spp. in the vineyard soil assay with GP. Similar growth results were also found in Trichoderma viride FRP3 isolated from Indonesian GP exposed soils4. Later, these authors reported the GP degradation capacity of this strain on soil and their promissory agriculture application3.
The biodegradation of GP, as for other organophosphorus compounds, depends on specific enzymatic and nonspecific enzymatic reactions. These processes occur via the secretion of phosphatase and other hydrolytic enzymes involved in the opening of phosphate bonds (P-0 and P-S)26. Genes encoding organophosphorus hydrolase and organophosphorus acid anhydrolase are expressed in several microorganisms when they are exposed to organophospho-rus compounds. This fact would induce the synthesis of the corresponding phosphatase. In addition, gene mutation and recombination are the most relevant genic processes that explain the microbial adaptation at contaminated sites17. This is consistent with several studies that have shown that the history of organophosphorus pesticide exposure would significantly improve the degradation rate28,25. In the same way, it has been suggested that the most effective degrada-tion process is related to functional microbial groups rather than to one microorganism47.
Conclusión
The evaluation of all data showed that the two Aspergillus spp., two Trichoderma spp., three Mucor spp. and three Sterilia spp. (78, 133 and 135) isolates had the highest EC50 and growth rate values in media supplied with the herbicide. These isolates showed good growth performance after a variable acclimation period, indicating the inductive character of the GP degradation process. These results provide valuable data for further studies about the metabolic capacity of these promissory fungal species isolated from agricultural soils. These fungi could be potential candidates for enhancing GP removal both in pure and mixed cultures.
Conflict of interest
The authors declare that they have no conflicts of interest.












 uBio
uBio 

