Leptospirosis is a bacterial disease that affects several species of animals and humans8. This neglected zoonosis has worldwide distribution; nonetheless, its occurrence in developing countries is higher, especially in tropical areas, where environmental conditions contribute to the endemic status of leptospirosis5.
The microscopic agglutination test (MAT) is the reference assay for the serological diagnosis of leptospirosis, detect-ing both IgM- and IgG-class antibodies11. The test is based on an antigen-antibody reaction and live samples of Leptospira spp. (serovars) are used as antigens8; for this purpose, the bacteria must be periodically seeded in a new medium for maintenance in the laboratory3. Although other serological automated or more modern techniques are available, such as ELISA (enzyme-linked immunosorbent assay) and LFA (lateral flow assay)7, MAT is still the most widely utilized tool.
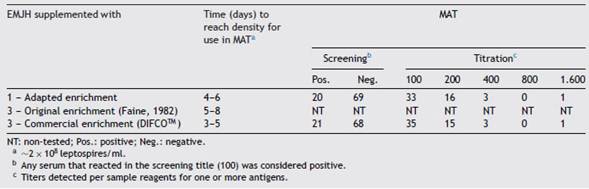
The EMJH medium (Ellinghausen-McCullough-Johnson-Harris) is commonly used in the cultivation of leptospires for performing MAT, and consists of a base supplemented with an enrichment traditionally composed of bovine serum albumin (fraction V), Tween 80, chlorides, sulfides and vita-mins. This enrichment can also be purchased commercially. However, due to the high cost of using this commercial enrichment routinely, many laboratories produce their own enrichment in accordance with the formulation described by Faine4. In our laboratory, we observed that some Leptospira strains did neither grow nor reach the ideal density for use in MAT (~2 x 108 leptospires/ml) when seeded in the EMJH medium with the enrichment produced by us using this formula. Thus, the purpose of this work was to adapt the original composition of the enrichment to provide the ade-quate growth of all strains used as antigens in our laboratory MAT panel.
The enrichments were prepared by weighing the ingredients in agreement with the desired volume, hydrating and sterilizing by filtration in a PES membrane (polyether-sulfone, 0.22 ^m). The final pH of the enrichments was enrichment; 2 - original enrichment4; 3 - commercial enrichment (DIFCOTM). This base was sterilized by autoclav-ing at 15 psi pressure and 121 °C for 15min and, after the medium reached a temperature of approximately 50-60 °C, the enrichments were added in the proportion of 10% (v/v) to the base medium.
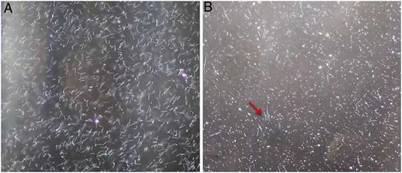
Figure 1: Leptospira interrogaos serovar Autumnalis grown in EMJH with adapted enrichment (A), and original enrichment (B), after 4 days of incubation. Red arrow indicates cells with a spirochete shape. Dark field microscopy - 20x.
For the functional test, we used 24 samples of Leptospira spp., encompassing reference and autochthonous strains isolated in Brazil (Supplementary material 1) which compose our MAT panel applied in the diagnosis of leptospirosis in animals.
Initially, a first passage was made from the strains stocked in Fletcher’s semi-solid medium. Each sample was seeded individually in tubes containing the EMJH medium supplemented with three different enrichments and incubated in a bacteriological incubator at 28 °C for 6-7 days for a first adaptation to the liquid medium (EMJH). Consecutive weekly passages, always at a ratio of 1/10 (v/v), were carried out in new tubes containing the EMJH medium with the distinct enrichments and incubated as described above. These passages were followed up until the growth of all the strains was macroscopically visible (similar to white smoke). Each strain was individually examined through dark field microscopic observation for cell viability (motility), contamination and auto-agglutination. The cell density was determined by counting, using a Petroff-Hausser chamber4. Cultures should ideally reach ~2 x 108 leptospires/ml for subsequent use in the MAT4 and the number of days for this to occur was taken into account.
The MAT was performed according to Faine et al.3, using three panels with 24 antigens, each grown in EMJH with the respective enrichments tested. Eighty nine (89) blood serums from domestic and wild animals were randomly selected from the laboratory serum bank. They were col-lected from 12 horses, 15 buffaloes, 13 bovines, 11 dogs, 9 sheep, 15 pigs, 4 peccaries (Tayassu pécari), 4 crab-eating foxes (Cerdocyon thous) and 6 ocelots (Leopardus pardalis). The results obtained with the three MAT panels were compared by descriptive statistics using frequency measures. Furthermore, we sent the adapted enrichment to be tested by five other laboratories that study leptospires in Brazil.
Using dark field microscopy, we did not detect any conta-mination or presence of auto-agglutination in all media used, and cell viability (motility) was preserved in all cultures. With regard to the growth of Leptospira strains, all of them grew in EMJH with the adapted and commercial enrichment; however, only 20 samples grew in EMJH with the original enrichment (Supplementary material 2). Two reference strains (Autumnalis and Panama) and two autochthonous strains (Guaricura and GR6) did not grow. This was one of the reasons that motivated the change in formulation of the original enrichment4, as we observed that glycerol appeared to be toxic for these samples, since microscopically the leptospires grown in this medium lost their spirochete shape, assumed a coccoid shape (Fig. 1), and died within days (data not shown). This was interest-ing, because glycerol was reported as a growth requirement for pathogenic Leptospira and used to decrease generation times for serovars Pomona and Canicola10.
Other reagents were added to the adapted enrichment formulation and we observed that antigens reached the ideal density for use in MAT faster in comparison to EMJH with the original enrichment (Table 1). Moreover, when the autochthonous strains were seeded in the medium with original enrichment without addition of glycerol, poor growth occurred, and they did not reach the recommended concen-tration for use in MAT. This situation was resolved the moment that these samples were seeded in EMJH with adapted enrichment.
Metals, minerals, and some metal traces, such as manganese and copper are important for the growth of lep-tospires and can be related to bacterial pathogenicity6. An increase in the concentration of sodium pyruvate (0.1 g/l) proved to be useful in the cultivation of fastidious strains of leptospires belonging to the Sejroe serogroup1, in which the serovar Guaricura is inserted. Other reagents such as cyanocobalamin8, sodium acetate and sodium bicarbonate2 have been shown to be essential and enhancers in the culti-vation of Leptospira spp.
MAT results using EMJH with the adapted and commer-cial enrichments were similar in both screening and titration (Table 2). Due to the fact that the MAT panel was not complete using EMJH with the original enrichment, as four strains did not grow, the technique was not performed. MAT panels can vary from laboratory to laboratory, as the inser-tion of strains representing most prevalent serogroups in the region is recommended11, with the consequent increase in the sensitivity of the test to detect leptospirosis-positive samples9.
In this work we did not aim to assess the costs for prepar-ing adapted enrichment. Nevertheless, the acquisition of commercial enrichment (DIFCO™ - 6 vials x 100 ml), in our reality (Sao Paulo, Brazil) can range from US$ 800.00 to US$ 1000.00, while the cost for the production of the same vol-ume of adapted enrichment (600 ml) is approximately US$ 300.00. It is also worth emphasizing that although the prepa-ration of enrichment in the laboratory is laborious, it can be a valuable alternative for laboratories at this time of financial crisis in many countries, including Brazil.
Despite the fact that an exact shelf-life has not yet been defined after preparation, we observed in our routine that for up to three months after being produced and stored in refrigeration, the enrichment has given us satisfactory results (data not shown).
Finally, all five laboratories that received the adapted enrichment approved its use in their laboratories. In our laboratory we optimized this enrichment for more than a year, until we achieved this final formula that gave us satisfactory results, similar to the ones obtained with the commer-cial enrichment. Thus, other laboratories that manipulate autochthonous strains different from the reference strains could also benefit from the use of this improved enrichment in the cultivation of the antigens applied in their MAT panels.
Funding
This work was financed in part by CAPES (Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior), Brasil -Finance Code [001] and by CNPq (Conselho Nacional de DesenvolvimentoCientífico eTecnológico) [420110/2018-6].
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgments
MBH would like to thank CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for the fellow-ship (CNPq 309146/2017-8). IBG and JFPC are grateful to CAPES (Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior) for the scholarship. We appreciate the fol-lowing laboratories for testing the adapted enrichment: Laboratório de Doencas Bacterianas da Reproducao - Instituto Biológico de Sao Paulo; Laboratório de Zoonoses e Saúde Pública, Instituto de Medicina Veterinária - UFPA; Laboratório de Zoonoses, FMVZ - Unesp Botucatu; Laboratório Especial de Desenvolvimento de Vacinas --- Instituto Butan-tan; Laboratório de Leptospirose - Instituto de Pesquisas Veterinárias Desidério Finamor.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ram.2021.03.002.












 uBio
uBio