Introduction
Intensive agriculture and livestock practices are leading to an increase in the punctual and diffuse discharges of contaminants of emerging concern, which may in turn lead to potential effects on humans and wildlife51. Currently, there is growing interest in the effect of pollutants on the intestinal microbiota, as it is closely linked to the metabolic and physiological functioning of its host organisms25. Although most environmental contaminants do not target the intestinal microbiota directly, some pollutants can interact with it through different pathways. It has been reported that the exposure of the intestinal microbiome to environmental pollutants can alter its composition and homeostasis, leading to metabolic disorders, nutrient malabsorption, and dysfunction of the immune system59. The disruption of the normal structure of intestinal microbiome communities (known as dysbiosis) may lead to an imbalance of bacterial species, which may in turn pose a risk to health38.
In a previous study, we described the enteric bacteria of R. arenarum tadpoles and defined them as reservoirs of bacteria of sanitary interest, which can be indicative of contamination28. Since aquatic organisms such as fish and amphibians have intestinal bacterial pathogens that may be resistant to antibiotics42, dysbiosis in these organisms may lead to the emergence of resistant pathogens23.
Thus, following the relationship between contamination, the environment, human health and antibiotic resistance26 pointed out by the WHO’s ''One Health’’ approach (https://www.who.int/news-room/questions-and-answers/ item/one-health), the effect of contaminants of emerging concern on freshwater organisms should be evaluated worldwide13. Over the past few decades, a high number of pharmaceutical residues (e.g. antibiotics) has been detected in surface, ground and drinking water. This fact alarmed the scientific community, especially due to the risk of bacterial acquisition of resistance16,27. Moreover, several studies have shown that antibiotics can affect the diversity of enteric bacteria and increase the risk of bacterial infections in wildlife and humans56,60.
Some of the antibiotics most commonly found in aquatic environments are fluoroquinolones46. One of the most widely prescribed fluoroquinolones to treat human and livestock infections is ciprofloxacin (CIP)15,37. Just as other fluoroquinolones, CIP interferes in DNA synthesis by inhibiting DNA gyrase and is active against gram negative and gram posi-tive bacteria8. Since high CIP concentrations (ranging from 16 ^g/l to 150 ^g/l) have been found in wastewater and residual effluents32, this antibiotic is considered an emerging pollutant in aquatic environments with the potential to spread antibiotic resistance55. CIP has been reported to produce dysbiosis in the intestinal microbiomes of humans10 and zebrafish (Danio rerio)44, and to increase fecal deposition in emaciated tadpoles36.
Other contaminants commonly found in several ter-restrial and aquatic ecosystems are pesticides. Among them, glyphosate (GLY), which is the most used herbi-cide worldwide4, has been found at high concentrations in aquatic environments54. This herbicide is an inhibitor of 5-enolpyruvylshikimate-3-phospate synthase, an enzyme present in plants and other organisms such as bacteria. GLY and other herbicide cocktails have been shown to affect microbial communities30. Moreover, it has been suggested that GLY can cause enteric dysbiosis, mainly affecting ben-eficial bacteria (beneficial/pathogenic imbalance)43.
Both glyphosate-based herbicides (GBHs) and CIP are present in surface water worldwide17. In crop-livestock systems, GBHs and CIP are found together and their accu-mulation in food crops represents a direct pathway for their insertion into the food chain and consequently, a human health risk17. Recently, our group described that the envi-ronmental exposure of amphibian tadpoles to a GBH and CIP can cause morphological and developmental abnormalities and thyroid malfunction9. Since amphibians are considered reliable models for the evaluation of toxic effects of pol-lutants and may provide insights into different biological processes7,31, the analysis of enteric bacteria of tadpoles may help to understand the impact of these pollutants on aquatic organisms. However, the effect of these pollutants on the tadpole intestinal microbiota remains unstudied.
The aim of this study was to analyze the toxic effects of commercial formulations of a GBH and CIP (both individ-ually and in mixture) on the enteric bacterial community of common toad (R. arenarum) tadpoles. Cultivable fast-growing bacteria with low nutritional requirements were identified by classical microbiological techniques and by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOFMS). Our hypothesis was that the exposure of tadpoles to a GBH and CIP would cause changes in the composition of their enteric bacterial communities.
Materials and methods
Treatments and samples
Surface egg strings of R. arenarum were collected from small temporary ponds situated in the natural floodplain of the Paraná River (31°11'31"S, 60°9'29"W), considered an unpolluted site9,14, with authorization of the Ministry of Environment of the Province of Santa Fe (Exp. No. 021010026248-0), Argentina. Egg strings of several ovipositions (50 cm of each string) were immediately transported in dechlorinated tap water (DTW) to the laboratory. The eggs and hatching embryos were acclimated under laboratory conditions for 48 h under a 12-h light/dark cycle in flasks with DTW at 24 ±2 °C. The tadpoles used in the bioassays were treated in accordance with the standardized experimental laboratory procedure of the American Society of Ichthyologists and Herpetologists2.
Tadpoles were exposed to a commercial formulation of a GBH (74.7% active ingredient, N-phosphonomethyl glycine and inert adjuvant quantum satis; Roundup Ultra-Max®, Monsanto© Argentina), and CIP (Sigma-Aldrich, Germany). Both chemicals were tested individually at nominal con-centrations considered relevant in the worst-case scenarios for natural lentic aquatic systems9,54: GBH = 2.5mg/l and CIP = 100 ^g/l, and in mixture (GBH-CIP) with 50% of each stock solution at the same concentration as in the individual treatments. The solutions were prepared using dechlorinated water, which was also used as a negative control. The assay was carried out following the procedures previously described in Cuzziol Boccioni et al.9. The solutions were totally renewed every 48 h.
After 14 days of exposure, intestinal tracts (n = 15 per treatment) were removed under sterile conditions, weighed, and pooled in three samples (one from each replica, consisting of 5 individuals each) due to the low tis-sue volumes. Pool samples were homogenized in 500 ^l of sterile peptone water (0.1% pluripeptone; 0.85% NaCl) using sterile glass beads (425-600 ^m in diameter) for the rupture of intestinal walls. Serial dilutions (up to 1/105) of homogenized intestines were plated onto nutrient agar plates (0.5% pluripeptone; 0.3% meat extract; 0.8% NaCl) and incubated at 37°C for 24h. To evaluate the quantitative differences between treatments, colonies were counted and expressed as colony forming units per gram of intestine (CFU/g).
Identification of enteric bacteria
A total of 60 CFUs were studied for each intestinal sam-ple (20 CFUs per plate were randomly selected from each replicate). Each CFU was re-suspended in 1 ml of nutrient broth medium (0.5% pluripeptone, 0.3% meat extract, 0.8% NaCl, 2% agar) and incubated at 35-37°C overnight. Each CFU was firstly identified by Gram staining and biochemi-cal tests including: triple sugar iron agar, citrate, indole, motility, urease, phenylalanine and lysine-iron (according to Lopardo et al. and Ochoa and Ochoa)29,35. The strains that showed discrepant results or were difficult to identify were identified by MALDI-TOF MS using the VITEK MS system (bioMérieux). MALDI-TOF-MS-based identification was also used to confirm the identification of 10% of the strains identified by classical biochemical tests. A total of 240 strains were identified by biochemical methods and 34 strains were identified by MALDI-TOF MS.
Data analysis
Data on morphological biomarkers are reported as the mean ±SD. Differences in the CFU/g of tissue among treatments were evaluated by the Kruskal-Wallis test followed by the Dunn post-hoc test. Plate count results are expressed as CFUs and were used to calculate CFU/g of tissue (intestine) as follows:
[No. colonies/ml plated/dilution factor]/[grams tis-sue/ml original homogenate] =No. colonies/g of intestine =CFUs/g tissue. Data are shown as log (CFU/g of intestine).
Alpha diversity parameters (Chao1 richness estimator, Dominance index, Shannon and Simpson diversity index) were calculated using the PAST 3.22 software19. Indices are expressed as the mean ±SD (from replicas). Taxa rich-ness is expressed as total genera in each treatment. The frequency of each taxon was calculated for each treat-ment and analyzed using the chi-square test to determine whether there were significant differences among treatments. MANOVA (Wilks’ lambda multivariate test statistic) was performed to determine whether there were significant overall differences in the diversity parameters of enteric bacteria among treatments. Subsequent univariate analysis of variance (ANOVA) followed by Dunnett’s post-hoc tests were performed for each parameter. Beta diversity analysis was performed to investigate the structural variation in the bacterial communities among treatments and then analyzed via principal component analysis (PCA) and unweighted pair-group method with arithmetic means (UPGMA) hierarchical clustering (adapted from Jiang et al.)24.
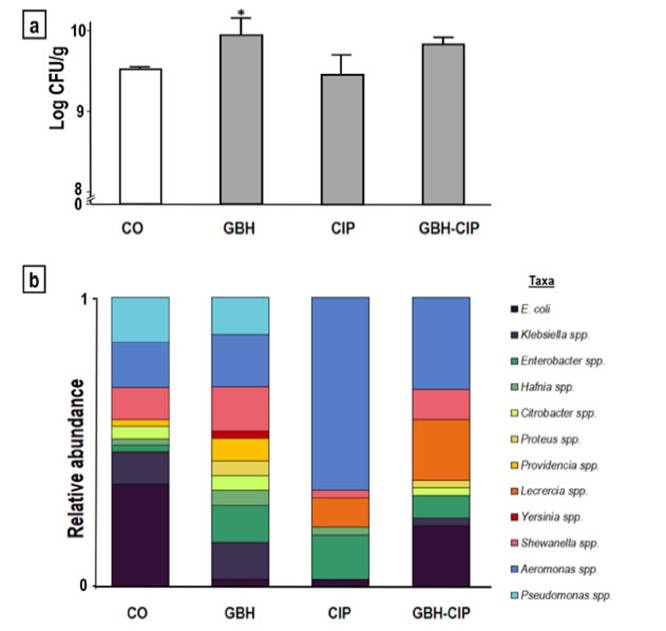
Figure 1: Comparison of enteric bacteria identified in samples treated with a glyphosate-based herbicide (GBH), ciprofloxacin (CIP) and their 50:50% mixture (GBH-CIP), with respect to control (CO). (a) Histogram of the plate count of colony-forming units (CFUs)/g of intestine, *significantly different from CO (Dunn post-hoc test p < 0.05). (b) Variation in taxa relative abundance in each treatment (cumulative frequencies of each genus over the total CFUs of each treatment, n = 60).
Data distribution was assessed for normality
(Kolmogorov-Smirnov test) and homogeneity of variances (Levene’s test) for statistical analysis. BioEstat software 5.03 and InfoStat software11 were used for statistical analysis. Significance was accepted as p < 0.05.
Results
The number of CFUs was significantly higher in GBH-treated samples than in control samples (increase of 4%, KW= 14.930, p <0.01; Fig. 1a). Alpha diversity parameters of enteric bacteria isolated from GBH-, CIP- and GBH-CIP-treated samples varied significantly with respect to control samples (MANOVAWilks’ Lambda % = 0.0041; F=4.84; p<0.01). In GBH-treated samples, richness (Chao estima-tor) and diversity (Shannon and Simpson indexes) increased with respect to control samples (F = 11.8, p <0.01 and F =29; p <0.01, respectively). In contrast, in CIP-treated samples, diversity markedly decreased and dominance significantly increased (F = 27, p <0.01; Table 1). In GBH-CIP-treated samples, diversity and richness Indices were similar to those of the control samples, but the relative abundance of some genera was different (Fig. 1b).

Table 1: Diversity Indices of enteric bacteria genera identified in samples from control (CO), treatments with glyphosate-based herbicide (GBH), antibiotic ciprofloxacin (CIP) and their 50:50% mixture (GBH-CIP).
In the control samples, the most predominant taxa were Escherichia coli, Klebsiella spp., Shewanella spp., Aeromonas spp. and Pseudomonas spp. In CIP- and GBH-CIP-treated samples, the relative prevalence of Aeromonas spp. increased. The increase in this genus explains the higher dominance obtained in the CIP treatment. In contrast, in GBH-treated samples, Aeromonas spp. dominance was lower (Table 1). Yersinia spp. and Proteus spp. were present only in GBH- and GBH-CIP-treated samples. The increase in taxa richness and diversity in GBH-treated samples was given by the higher number of taxa identified, including the two addi-tional ones not found in the control samples.
The results of the PCA showed that the replicas from each treatment were closer to each other than the samples of the other treatments, except for the GBH-treated repli-cates. GBH replicates were scattered along the Y axis and very close to the control samples in the X axis. Figure 2a shows that the control, GBH-, CIP- and GBH-CIP-treated samples varied in taxa between the component axis. Most of the variation was represented in the X axis (XPC1 = 59.2%), where enteric bacteria from control samples were closely related to those of the GBH- and GBH-CIP-treated samples, whereas enteric bacteria from CIP-treated samples were dis-tant due to differences in the taxa composition. Vectors representing each taxon were in agreement with the param-eters of diversity and relative abundance, and allowed a more detailed comparison of the variation of each of them among treatments. Several vectors were located close to the GBH-treated samples, corresponding to the previously described increase in diversity. Vectors of Pseudomonas spp., Shewanella spp. and Citrobacter spp. were located between the control and GBH-treated samples, since their relative proportions remained similar in both treatments. Vectors of Providencia spp., Klebsiella spp. and Hafnia spp., which were close to the GBH-treated samples, denoted an increase in their relative frequency in this treatment. Vectors of Serratia spp. and Proteus spp. were also located close to the GBH-treated samples and far from the others since they were only identified in this treatment. The vector of Aeromonas spp. was located markedly close to the CIP-treated samples, denoting an increase in relative abundance and dominance in the CIP treatment. The vector of Lecrercia spp. was located close to the GBH-CIP-treated samples, and, among the taxa identified, was the one that varied the most in relative abundance with respect to the control samples.
The hierarchical clustering tree showed that replicates from CIP-treated tadpoles clustered together into one group by similarity of identified taxa, and replicates from GBH-CIP-treated tadpoles clustered into another group. All replicates from control and one replicate from the GBH treatment clustered together into another group. The two remaining replicates of the GBH treatment clustered into a different group close to the control one (Fig. 2b). Overall, according to the UPGMA analysis based on the relative abundance of taxa identified in each treatment, the GBH treatment was closely related to the control, whereas the GBH-CIP treat-ment was closer to GBH and the control than to the CIP treatment.
Discussion
In aquatic environments that receive effluents or lixiviate from agroecosystems and swine, chicken, and cow feed-lots, pesticides are likely to be found together with human and veterinary antibiotics41. The interaction between pes-ticides and antibiotics and their effect on enteric bacteria of tadpoles have rarely been studied. This research showed that GBH and CIP, both individually and in mixture, altered part of the cultivable enteric bacterial communities of tad-poles of R. arenarum, an autochthonous species considered a sentinel of environmental health5.
In this study, most of the enteric bacteria identified belong to Gram (-) bacteria of the family Enterobacteri-aceae, Aeromonas spp. and Pseudomonas spp. Although the composition of the gut microbiota can vary depending on many factors (such as, temperature, pH, conductivity and inter- or intraspecific variation)23,49, the taxa identified will depend on the method of isolation and identification. In most recent research studies on the variation of the intestinal microbiota of amphibians in response to contaminants, the identification relied on DNA isolation and sequencing directly from the intestine, and included a broader reper-toire of taxa analyzed on a larger scale13,23,57. In this regard, Lozano et al.30, explained that as the cultivable strains of the rat gut microbiota are a minor part of the total gut microbiota, the effects of GBH on the microbial commu-nity would be less likely to be detected than in the whole community by the sequencing method. In contrast, other authors have claimed that 16S sequencing should not be used to study the ''dominance’’ of certain higher-order taxa over others (e.g., Bacteroidaceae vs. Enterobacteriaceae) or to determine whether taxa are absent from a sample without first benchmarking a high correlation between these obser-vations and the gold-standard of culture, since this method could mean very little at the individual population or species level33. In our study, the strains were isolated from plate culture of the intestinal content, and thus only those that grew on the plate were studied. However, the variation of the taxa identified by culture may be the starting point for further studies to deepen on the effects of contaminants on the microbiota on a larger scale33.

Figure 2: Beta diversity (variation in taxa composition) of enteric bacteria among samples from the different treatments: control (CO), glyphosate-based herbicide (GBH), ciprofloxacin (CIP) and their 50:50% mixture (GBH-CIP). (a). The principal components analysis biplot shows the patterns of separation for treatments based on different predominant taxa (genera). (b) Hierarchical clustering (unweighted pair-group method with arithmetic means) of replicates of treatments based on taxa composition (genus level).
The bacterial genera found in the microbial communi-ties of the control tadpoles coincided with those described in pioneering works in the same species as well as other which used the same culture methodology20,28. E. coli, the most frequent taxon identified in the control group, was also reported by Martinson et al.33, in their culture-based eval-uation as the most dominant Enterobacteriaceae in healthy human feces. In our study, the enteric bacteria isolated from GBH-treated samples were different from those found in the control ones. In GBH-treated samples, the genera richness Indices increased considerably and the dominant taxa (i.e. E. coli) decreased. GBHs have been reported to disrupt the intestinal microbiota of animals living near agricultural sites such as water fleas (Daphnia spp.)47. GBHs can also alter the diversity of soil microbes and enhance the presence of certain species which perform less efficiently in normal conditions21. In general, it is agreed that diversity is given by the variation in environmental conditions and availabil-ity of nutrients18. Some Gram (+) and Gram (-) bacteria use GLY as a source of carbon, nitrogen and phosphorus52. In this sense, the dysbiosis observed in the enteric bacteria present in GBH-treated samples given by an increase in the diversity and evenness of taxa could be related to the fact that GLY positively influences bacterial growth. Other alterations in the normal conditions of the intestinal lumen due to xenobiotics such as GLY could also contribute to a shift in the composition of the microbiota. Furthermore, it has been suggested that the mucous layer of the intestine of mammals is affected by pollutants30.
Another explanation for the shift in the composition of enteric bacteria in GBH-treated samples may be related to the ability of some bacteria to transform GLY48. Some bacteria transform GLY into aminomethylphosphonic acid (AMPA) by the enzyme glyphosate oxidoreductase and use either this metabolite or GLY to obtain phosphate for their metabolism by C-P bond break down21. Thus, it has been suggested that GBH application may induce an artificial selection that stimulates bacteria able to degrade the herbicide53. In agreement with this, some of the taxa that were here observed to be increased in the GBH treatment, such as Enterobacter spp. and Providencia spp., use GLY as a sub-strate or have traits associated with GLY degradation34. From an ecological point of view, the decrease in some bacterial species could leave ecological niches available for others6. However, further studies are needed to gain further insights into the effects of different taxa of enteric bacteria on the imbalance between beneficial and pathogenic bacteria in both wildlife and humans.
With regard to the quantification of CFU/g, the GBH-treated samples showed a higher number of CFU than the control samples. This effect could be related, among vari-ous factors, to host transcriptional changes enriched for lipid and carbon metabolism, as suggested by Suppa et al.47. In addition, studies on the dynamics of bacterial communities of the soil and rhizosphere have associated the increase in the abundance of fast-growing bacteria with the availability of carbon compounds in the presence of GLY21. There-fore, the increase in the number of CFUs/g observed in this study in the GBH-treated samples may be due to the increase in those taxa capable of degrading GLY. Because of GLY degradation, the production of AMPA would increase together with its potential risks to the health of host animals and humans (e.g. impairment of DNA reparation and mRNA synthesis)1.
With respect to antibiotics, studies on their effects on the intestinal microbiota are mostly focused on human health and warn about the consequences of prolonged treatments and permanent loss of certain fundamental taxa for the maintenance of a healthy intestine39. However, it is also important to pay attention to the possible effects of drug residues on the bacterial communities of non-target organ-isms, since these are frequent in water bodies36. In our study, the CIP treatment induced dysbiosis on enteric bacteria by the reduction of taxa diversity and increased dominance of a single genus. Aeromonas spp. represented more than 50% of the relative taxa abundance on enteric bacteria from CIP-treated samples, suggesting their tolerance to the antibiotic. This result is consistent with those of other stud-ies that reported multidrug-resistance (including CIP) of Aeromonas spp. from wild animals12.
The presence of dysbiosis in CIP-treated samples can lead to serious consequences on both wildlife and humans, since some of the Aeromonas spp. (e.g. Aeromonas veronii and Aeromonas hydrophila) identified in this study have been associated with several diseases in humans and fish22. Skwor et al.45, warned about the risk of emergence of resistant Aeromonas spp. in the environment and organisms, due to the overuse of antibiotics. In addition, Leclercia spp. was another taxon that suggested the presence of dysbiosis in CIP-treated samples, since it did not appear in the control or GBH-treated samples. In Leclercia spp. strains isolated from farm poultry intestinal tracts, Yehia58 reported multi-ple resistance to antibiotics (including CIP) and enhanced the health risks of inadequate use of a wide spectrum of antibiotics for different uses.
In the present study, the richness and diversity Indices obtained for enteric bacteria isolated from GBH-CIP-treated samples were similar to those of the control samples, but the taxa composition was different. Some genera present in control samples, such as Klebsiella spp. and Pseudomonas spp., were decreased or absent in the GBH-CIP-treated sam-ples. Moreover, some of the trends observed for individual pollutant treatments were also observed in the GBH-CIP-treated samples: an increase in Enterobacter spp. and the presence of Proteus spp. (as in the GBH treatment), and an increase in Aeromonas spp. and the presence of Leclercia spp. (as in the CIP treatment). It is more than clear that the interaction of both pollutants influences the structure of the enteric bacterial community. The changes observed in this work were only on fast-growing cultivable bacteria with low nutritional requirements, which represent only a small percentage of the total intestinal microbiota. It is important to consider other potential effects on other mem-bers of the gut microbiota that also intrinsically influence the bacterial community dynamics. Although the results of the present study are preliminary and limited to a small portion of the whole enteric microbiome, they confirm the susceptibility of at least part of the enteric bacteria from an autochthonous model organism to different types of pollutants, both individually and in mixture. The latter is particularly important because the pollutants studied are likely to be found together in the environment40. Pollu-tants of emerging concern have been described to affect enteric bacteria and to lead to a great imbalance in the host’s intestinal microbiota50. In the last years, there has been a growing interest and concern about the diversity and structure variation of the intestinal microbiota due to changes in environmental conditions and pressures to under-stand its complex symbiotic relations with the host’s life and the bacterial resistance due to exposure to antibiotics such as CIP9. To our knowledge, this is the first preliminary report on the disruption of part of the enteric bacteria of a non-conventional model organism by a mixture of an antibiotic and an herbicide. Further studies are needed to elucidate how real-life scenarios with complex pollutant mixtures can affect the whole tadpole microbiota and, ultimately, aquatic life and human health.
Conclusión
Cultivable fast-growing bacteria with low nutritional requirements obtained from the gut of R. arenarum tadpoles were disturbed after exposure to commercial formulations of a GBH and CIP, individually and in mixture. The changes observed in the diversity and abundance of Enterobac-teriaceae and closely related taxa when exposed to the pollutants studied suggest a dysbiosis effect that may lead to additional physiological problems through alterations in the normal functioning of the intestinal microbiota, including metabolic activities related to nutrients and energy recov-ery. Further studies are needed to gain further insights into the effects of different taxa of enteric bacteria on the imbalance between beneficial and pathogenic bacteria, especially those of health interest, in both wildlife and humans.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
This study was supported by National Agency for Promotion of Science and Technology (PICT No. 1069).
We thank Laboratorio de Micología y Diagnóstico Molecular for lending its facilities and equipment. We also acknowledge Candela Martinuzzi and Andrés Attademo for laboratory assistance.
Received 28 February 2022
accepted 20 August 2022
Available online 20 January 2023












 uBio
uBio 

