Introduction
Haemophilus influenzae (Hi), a pleomorphic gram negative coccobacillus, is a strictly human pathogen colonizing the upper respiratory tract and causing invasive disease such as meningitis, pneumonia, bacteremia, arthritis, cellulitis, epiglottitis, sepsis and noninvasive infections, namely sinusitis and otitis media20,45.
Strains are classified according to the presence or absence of a capsule. Six distinct encapsulated types have been identified and assigned letters from a to f, based on the chemical structure of the polysaccharide capsule. Non-encapsulated forms (NCHi) are also known as non-typable strains (NTHi). In addition, capsule-deficient mutant strains have been identified (Hib(), which despite having a type specific capsular gene, do not exhibit the corresponding polysaccharide. These can be a source of vaccine failure29.
Among the encapsulated strains, type b is the most virulent. Before routine immunization against Hib, this serotype accounted for 80% of all invasive infections and was the most common cause of bacterial meningitis in children under the age of 530. After the incorporation of Hib vaccines into the National Immunization Programs (NIP) in the early 1990s, the incidence of invasive disease as well as pharyngeal carriage declined dramatically, resulting in herd immunity17,23,30,37,40,47. At the same time, several studies showed increased rates of disease caused by NTHi and other non-b encapsulated strains (mainly a and f)10,19,22,42.
NCHi is a major cause of invasive infections in young infants ((20 weeks of age) and adults ((65 years of age), often producing pneumonia and bacteremia, without apparent source. Case fatality rates range between 10 and 20%18,38,44. In neonates, sepsis leading to severe disease and death is observed, and in pregnant women, septic miscarriage8,9. Invasive diseases due to NCHi generally occurs in infants with comorbidities and is associated with high fatality rates and chronic sequelae39. Non-invasive infections (otitis media, sinusitis and bronchitis) can develop in healthy individuals.
In Argentina, before the incorporation of a quadrivalent conjugate Hib vaccine into the National Immunization Program in 1998 (as a 3 dose primary schedule at 2, 4 and 6 months of age, and a booster dose at 18 months), invasive Hib disease was a leading cause of death and chronic sequelae in children. A whole cell pertussis pentavalent conjugate vaccine was subsequently introduced in 2005, resulting in a significant decline in the incidence of Hib meningitis, from 1.1 cases per 100000 inhabitants before immunization, to 0.1 cases per 100000 in 20065.
During the post vaccination period 2005-2010, laboratory surveillance detected the emergence of invasive infections due to NCHi and, to a lesser degree, of capsular types b and a. Subsequently, in 2010, the relative frequency of type b increased significantly10. In addition, the National Surveillance System observed progressive rise in Hib invasive infections, reaching a peak incidence of 0.3 per 100000 inhabitants in 2015; 60.7% of cases had received 3 or less vaccine doses21.
Based on this evidence, the objectives of this study were: to determine the proportion of Hi capsular types from isolates causing invasive disease in different age groups between 2011 and 2019, to identify Hib clones circulating between 2011 and 2015 and to compare circulating clones observed during the periods 2011-2015, to those detected between 1997 and 1998 (prevaccination-transition period).
Materials and methods
A cross-sectional, observational and analytical study was conducted. A total of 1405 Hi strains collected from children and adults with invasive Hi disease between January 1st, 2011 and December 31st, 2019 were analyzed at the National Reference Laboratory. One hundred and thirty hospitals and health centers located in 22 provinces and in Buenos Aires City, belonging to the National Laboratory Network for Meningitis and Acute Bacterial Respiratory Infection, contributed isolates. Invasive disease was defined as the isolation of Hi from a normally sterile site (blood, cerebrospinal, pleural, synovial, and other fluids). Strains were subcultured onto brain heart infusion agar containing 10% horse blood supplemented with 1% Isovitalex (BBL), and incubated in 5% CO2 atmosphere, at 37C for 18-24h.
Bacteria were identified at genus and species level using conventional biochemical tests; the PCR technique was used to detect the following genes: omp encoding the genus and species specific outer membrane protein (OMP) P213, bexA encoding the capsulation-associated protein48, and capsule type-specific cap types a, b, c, d and f, as previously described by Falla et al.12 For type e cap, modified primers were used with the following sequences: e10: 5(-GTGAATTTGAAGTCGTCCATAG-3(, e11: 5(-GTCTGCTTAGGGGTTTCTTCA-3( (in house).
Molecular epidemiology testing was carried out using pulsed field gel electrophoresis (PFGE) and multilocus sequence typing (MLST).
PFGE was performed on 92 of 319 Hib isolates from the post vaccination period 2011-2015 (due to the large number of samples, only 1 of 4 was systematically selected to cover the whole period), and on 30 isolates from the prevaccination-transition period 1997-1998 (isolates viable at time of study). Tests were conducted in accordance with previously described procedures34. Digestion with restriction enzymes using SmaI (Fermentas) was carried out for 18h at 25C. Fragments were separated in 1% agarose gels (Bio-Rad, Hercules, Calif), in 0.5( TBE buffer on a BioRad CHEF-DR III System, at 14C. Parameter setting was 6V/cm, initial pulse time 1s and final pulse time 20s, run time 21h. Gels were stained with GelRed( (Biotium) for 30min. Salmonella ser. Braenderup (H9812) was used as a reference standard, as described previously11. Gel images were captured under UV light using Gel Doc Quantityone (Bio-Rad) software. Molecular profiles were analyzed using BioNumerics version 7.6.3 (AppliedMaths, Kortrijk, Belgium). Clonal relatedness was estimated by the percentage of band sharing, applying the Dice similarity coefficient (position tolerance and optimization were set at 1%); dendrograms were generated based on the unweighted pair group method with arithmetic mean (UPGMA). Molecular profiles obtained by Smal-PFGE were clustered above 80% similarity.
Sequence type was determined by MLST on a subgroup of 14 Hib isolates from the 1997-1998 period and 36 from the 2011-2015 period, selected according to cluster representative PFGE profiles as described by Meats et al.28 Briefly, 7 intrinsec or housekeeping genes representing the species (adk, atpG, frdB, fucK, mdh, pgi, and recA) were amplified by PCR, sequenced and compared to an international sequence database (https://pubmlst.org/organisms/haemophilus-influenzae/) to identify the combination of 7 alleles defining an unique sequence type. Relatedness between MLST isolate profiles was established using goeBURST software for an unrooted tree-based representation of the relationship14. A tree-cut off equal to 5 was set.
Statistics
Univariate analysis was conducted independently for each variable and results were expressed as absolute and relative (%) values. Absolute and relative frequency distributions, and measures of central tendency and dispersion (median and interquartile range, IQR) were estimated. To compare the proportion of capsular type b in 2011, 2015 and 2019, the test for homogeneity for independent proportions was performed using contingency tables considering the proportion of positive diagnoses in each sample and the Chi-square test (2) was applied. For multiple comparisons between years, confidence intervals for the difference in proportions were estimated and Bonferroni correction applied. When the comparison between capsular type b proportions was significant, the prevalence ratio (PR) was calculated and the respective 95% confidence interval (95% CI). To assess the association between capsular types and clinical presentation, and capsular types and age group, we used the Chi-square test of independence and the Phi coefficient was calculated as measure of association. In all cases, significance level was set at 5%. Epidat 3.1 and Epidat 4.1 software was used for data analysis.
Results
Between January 1st, 2011 and December 31st, 2019, a total of 1405 Hi strains, isolated from 1405 patients with invasive disease derived from the National Laboratory Network for Meningitis and Acute Respiratory Infection, were analyzed. In 1383 of 1405 patients, age ranged from 0 to 1085 months (90 years), median age was 10 months (interquartile range 19 months); in 22 cases, age information was missing. Most patients with Hi infection were under 1 year of age, 52.9% (732/1383) as follows: <1 month, 4.8% (n=66), 1 month, 4.3% (n=59), 2-3 months, 9.3% (128), 4-5 months, 11.7% (n=162) and 6-11 months, 22.9%, (n=317). Age distribution in older patients was: 12-23 months, 18.3% (253/1383), 24-59 months, 11.6% (161/1383), 5-14 years, 6.9% (95/1383), 15-59 years, 6.3% (87/1383) and (65 years, 4.0% (55/1383). Over 80% of isolates came from children under 5 years of age.
Most common clinical presentations included: pneumonia, 34.5% (485/1405), acute meningitis 28.9% (406/1405) and bacteremia, 25.8% (362/1405). Less frequently, cellulitis, 4.1% (58/1405), arthritis, 2.4% (34/1405), and others, 4.3% (60/1405).
In children <1 year of age, meningitis was more frequent, 35.8% (262/732) followed by pneumonia, 29.5% (216/732) and bacteremia, 23.5% (172/732). Pneumonia was most prevalent in individuals aged 12-23 months, 42.7% (108/253), 24-59 months, 42.2% (68/161) or over 65 years, 56.4% (31/55). Bacteremia predominated in children 5-14 years, 40.0% (38/95) and adults 15-64 years, 37.9% (33/87). Cellulitis and arthritis occurred more often in children under 2 years of age (Fig. 1A Fig. 1).

Figure 1: Panel A. Distribution of clinical presentations by age group (proportions). Argentina, 2011-2019. Panel B. Evolution of the annual proportions of H. influenzae capsular types. Argentina, 2011-2019. nc: non-encapsulated.
With respect to capsular types from 1405 isolates, the most common were NCHi, 44.5% (n=625) followed by type b, 41.1% (n=577), a, 10.0% (n=140), f, 2.3% (n=32), d, 0.9% (n=13), e, 0.9% (n=13) and c, 0.4% (n=5). Notably, one of the b isolates was a capsule-deficient mutant strain (b() reported from a patient with pneumonia.
We observed a significant association between capsular types and age. Encapsulated strains were associated with infants in the age group 1 month to 4 years while NCHi were associated with infants 0-1 month old and with ages 5 years and older (2=491.21; p<0.001; Phi=0.39). The most frequent encapsulated types in all age groups were b and a; b prevailed in children under 5 years of age (Table 1 Table 1). As regards the distribution of capsular types and clinical presentation, significant associations were found between type b and meningitis (2=228.19; p<0.001; Phi=0.40), cellulitis (2=33.34; p<0.001; Phi=0.15) and arthritis (2=10.17; p<0.001; Phi=0.08); and between NCHi and pneumonia (2=72.21; p<0.001; Phi=0.23), bacteremia (2=59.74; p<0.001; Phi=0.21) and neonatal sepsis (2=31.21; p<0.001; Phi=0.15) (Table 2 Table 2).
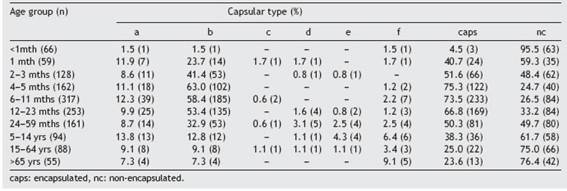
Table 1: Distribution of capsular types of H. influenzae according to age group in Argentina, 2011 -2019.
Table 2: Distribution of capsular types of H. influenzae according to clinical presentation in Argentina, 2011 -2019. Clinical presentation (n) Capsular type (%)
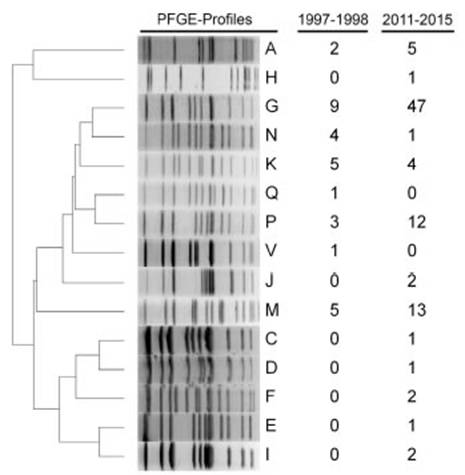
Figure 2: Representative PFGE profiles of circulating H. influenzae type b clones in the periods 1997-1998 and 2011-2015 in Argentina. The columns include the number of isolates grouped in each pulsotype, by period.
Among neonates, the most common diagnoses were: sepsis, 42.4% (28/66), bacteremia, 31.8% (21/66), pneumonia, 15.2% (10/66), meningitis, 7.6% (5/66), arthritis, 1.5% (1) and peritonitis, 1.5% (1).
During the study period, year 2015 exhibited the highest proportion of type b, 50% (96/192). The test for homogeneity for proportions of type b in the years 2011-2019 was significant ( 2 =13.6; p=0.001). We calculated 95% CIs (Bonferroni corrected) for the differences in proportions between 2011-2015 (0.04; 0.33) and 2015-2019 ((0.293; (0.035) and both were significant, showing a significant increase in the proportion of type b in 2015 (96/192) compared to 2011 (29/93) and a significant decrease in 2019 (51/152) compared to 2015. The PR (and 95% CI) of type b in 2015 with respect to 2011 was 1.6 (1.14; 2.23), and in 2019 with respect to 2015, 0.67 (0.51; 0.87) (Fig. 1B).
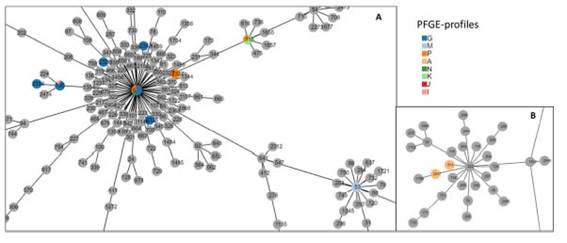
To compare Hib clones circulating during the period of high type b proportion to those of the prevaccination-transition period, we established genetic relatedness between a random sample of 92 isolates from 2011 to 2015, and 30 isolates from 1997 to 1998. PFGE analysis showed that strains were grouped into 15 pulsotypes, 8 present in the prevaccination-transition period, and 13 in the post vaccination period, 6 of which were present in both, namely A, G, K, M, N and P (Fig. 2 Fig. 2). Pulsotype G predominated in both periods, prevaccination-transition, 30.0% (9/30) and post vaccination, 51.1% (47/92), followed by M, 16.7% (5/30) and 14.1% (13/92), P, 10.0% (3/30) and 13.0% (12/92) and K, 16.7% (5/30) and 4.3% (4/92), respectively. Clones G, M, P and K accounted for 73.3% and 82.6% of all strains in the pre and post vaccination periods, respectively. MLST of 14 pre vaccination and 36 post vaccination strains, showed that clones G, M, P and K belonged to sequence type (ST) 6, or its simple or double locus variants (SLV, DLV) (Fig. 3 Fig. 3 and Table 3 Table 3). Ten M pulsotypes belonged to ST53, a DLV of ST6. Three A pulsotypes, identified as ST913 (n=2) and ST2347 (n=1), were the only isolates not related to ST6. Geographic clustering of clones was not observed.
Eight Hib strains were collected from patients who were fully vaccinated (receiving 3 primary doses at 2, 4 and 6 months of age and a booster at 18 months). These strains were 3 G, 3 P, 1 M and 1 I.
Discussion
This study assessed results from the laboratory surveillance for Hi strains recovered from individuals presenting invasive infection in Argentina during the years 2011 to 2019. Molecular typing of a sample of isolates from the period 2011-2015 showed an increase in the proportion of type b. As observed in a prior study carried out in 2011---2015, most strains were derived from patients under 5 years of age, and the most common clinical presentations were pneumonia, acute meningitis and bacteremia10.
Non-encapsulated Hi predominated (44.5%), followed by type b (41.1%) and less frequently type a (10%). Compared to the period 2005-2010, we observed a significant increase in the proportion of type b, due to a decrease in NCHi10. After the incorporation of Hib vaccine to NIPs in many countries, invasive disease due to type b has declined and NCHi and other capsular types have emerged, exhibiting variable geographic distribution. Some countries in Latin America, such as Paraguay and Colombia, have found similar results to those of our study, type b being the most frequent encapsulated type followed by types a and f24,33. H. influenzae type a (Hia) has emerged as an important cause of pneumonia, meningitis and septic arthritis in Alaska, North of Canada and other countries with indigenous populations, particularly in young children under 24 months of age3,6. In this study, Hia also affected children under 2 years of age most often, causing meningitis, pneumonia, bacteremia and less frequently cellulitis and septic arthritis.
While encapsulated types prevailed in children 1 month to 4 years of age, NCHi was more common in neonates, children aged 5 years or older and adults. Regarding clinical presentation, sepsis was most frequent among neonates and pneumonia among the elderly ((65 years), both associated with NCHi as reported in other studies. Sepsis in neonates is associated with premature labor, early-onset infection (<48h), long term sequelae and high mortality rates9. In adults, NCHi causes lower respiratory tract infections and pneumonia, particularly in individuals with underlying pathologies36.
Between 2011 and 2015, we observed a significant increase in the proportion of type b isolates, from 31.2% to 50%. Likewise, data from the National Surveillance System reported a rise in the incidence of invasive Hib disease, from 0.2 cases per 100000 inhabitants in 2014, to 0.3 per 100000 in 2015. During the period 2013-2015, the incidence rate doubled in children under 5 (from 3.1 to 6.3 cases per 100000 children <5 years) primarily affecting children under 1 year of age. According to data from the National Ministry of Health for this time period, the median age of children affected was 8 months (CI: 5-13) (67% <12 months and 90.4% <24 months) and the most common clinical presentations were meningitis (55.5%), pneumonia (17.1%) and bacteremia (8.8%), with no significant differences between each of the 3 years21. The disease did not exhibit a seasonal pattern, and no clusters of cases were observed. Average national vaccine coverage rates for third and booster doses were 93.9% (range: 93.8-94.1%) and 79.1% (range: 73.5-83.5%), respectively4. However, 60.7% of cases with invasive Hib disease had received 3 doses or less, reflecting delays in vaccination programs. Vaccine efficacy against invasive Hib disease in children under 1 year of age, as estimated by the Orenstein method, was 95.2% in 2013 and 91.8% in 2015.
Infections occurring in children with complete primary Hib vaccination and/or booster dose were unexpected; however, countries such as the United Kingdom, the Netherlands and Gambia have also reported reemergence of invasive infections after full vaccination2,25,32,35. In the UK, an increase in disease rates was observed between 1999 and 2002 in children 0-4 years of age associated mainly with a decline in vaccine-induced immunity in 1-4 year olds31,41. The reasons for vaccine failure were linked to accelerated 3 dose vaccination schedules (at 2, 3 and 4 months of age) since longer intervals are known to be more immunogenic26, lack of a booster dose and use of combined vaccines containing acellular Bordetella pertussis components, which are less immunogenic against Hib27. Invasive Hib infections were caused by a clonal type with low genetic diversity, rejecting the idea that the increase in cases could have been due to adaptive changes in Hib strains2. In the Netherlands, the Dutch vaccination program included a booster dose at 11 months of age plus a whole-cell pertussis combined vaccine. In all age groups, invasive Hib infections increased to levels seen before the introduction of the vaccine. Molecular surveillance studies showed strains isolated from children younger than 4 years of age in the pre vaccination era presented low genetic diversity, while higher levels of diversity were observed in the post vaccination era. Conversely, in children older than 4 years, genetically diverse strains were observed during both periods. These findings suggest that in the Netherlands, reemergence could have been caused by increased circulation of Hib in individuals older than 4 years of age, particularly adults, and that young children no longer constituted Hib reservoirs, but were infected by adults carrying genetically diverse strains35,43. In Gambia, the lack of booster doses most probably explained the increased incidence of invasive infections25.
Unlike the situation in the UK and other countries, in Argentina, the Hib vaccine schedule comprises 3 primary doses at 2, 4 and 6 months of age and a booster at 15-18 months, using a combined pentavalent whole-cell B. pertussis vaccine.
In this study, we looked for the emergence of a hypervirulent clone that could have caused the increase in Hib cases in Argentina during the 2011-2015 time period. PFGE analysis of type b strains showed 4 clones predominating in both study periods, 1997-1998 and 2011-2015, pulsotype G was the most frequent (30% and 51.1% in the pre and post vaccination periods, respectively). Fully vaccinated patients (having received 4 doses) presented the same clones as those found in the rest of the population. The most common pulsotypes belonged to ST6, or one of its single or double locus variants, which is usually associated to invasive Hib infections worldwide7,16,19,35. Therefore, we did not identify a specific clone causing increased type b infections in Argentina.
Several countries have observed a rise in the incidence of invasive Hib disease, years after the introduction of Hib vaccines to their NIP46. Various factors can account for the reemergence of this infection, namely a decline in herd protection due to suboptimal vaccine coverage rates, reduced antibody titers in children lacking a booster dose, emergence of more virulent transmissible strains and use of different types of vaccines15. In Argentina, increased rates of invasive Hib disease can be attributed to multiple factors. Having ruled out aspects related to the emergence of specific clones, we believe disease reemergence could be associated with irregular vaccine coverage rates, delayed vaccination schedules, non-compliance with booster doses generating insufficient herd immunity, and use of different vaccine components leading to programmatic errors1. This highlights the need to develop national strategies to optimize vaccination coverage, among other policies, ensuring that all children in the country are adequately protected against life-threatening infections.
Figure 3: PHYLOViZ analysis showing the genetic relationship among the global collection of sequence types (STs) of H. influenzae.The population snapshot is focused on Argentinean STs. Dots (STs) are highlighted in colors according to the PFGE profiles reference.Gray dots referred to ST presented in the H. influenzae MLST database. Panel A centers to ST6 and related STs; panel B centers toST222 and related STs.
Laboratory surveillance revealed that the proportion of type b decreased significantly starting from 2016, reaching 33.6% in 2019. These results concord with data from the National Disease Surveillance program showing a decline in invasive Hib disease incidence rates from 2016 to 2018-2019, when rates stabilized at 0.1 cases per 100000 inhabitants, similar to values reported in 2013 (information provided by Dirección de Control de Enfermedades Infecciosas, Ministry of Health, unpublished data).
Table 3: Association of pulsotypes and sequence types of H. influenzae type b isolates from the periods 1997---1998 and 2011---2015 in Argentina.
One of the limitations of this study is that it was based on passive surveillance, as incidence data were unavailable. Nonetheless, results regarding the annual proportion of type b isolates coincided with increased disease incidence rates reported by the National Epidemiologic Surveillance System21, most likely because the National Reference Laboratory received samples from patients of all ages nationwide.
The main strength of this study is that it was the first to identify predominant STs associated with invasive Hib disease in Argentina.
In conclusion, it is crucial to conduct enhanced epidemiological surveillance of invasive Hib disease in order to detect changes in circulating capsular types, thus contributing to evidence-based decision making for disease prevention and control.
Funding
This work was supported by the regular federal budget of the National Ministry of Health of Argentinaand the FOCANLIS-NRU</ce:grant-sponsor>: 1709, 2017, to A.E. from the ANLIS ``Dr. Carlos G. Malbrán''. The funders had no role in the study design, data collection or analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest
The authors declare that they have no conflicts of interest.
National Surveillance Network, Argentina
Hospital de Pediatría ``Dr. Juan P. Garrahan''-Claudia Hernández, Alejandra Blanco, Vanessa Reitjman; Hospital General de Agudos ``Donación Francisco Santojanni''-Claudia Alfonso; ``Sanatorio Franchin''-Claudia Etcheves; ``Sanatorio Trinidad Palermo''- Débora Stepanik; ``Hospital Italiano''-Graciela Greco, María Ángeles Visus; ``Hospital de Niños ``Dr. Ricardo Gutiérrez''-Marisa Turco, Adriana Procopio, Miryam Vázquez; ``CEMIC''-Mariela Soledad Zarate; ``Hospital Británico''-Marta Giovanakis; ``Hospital de Niños ``Pedro de Elizalde''-Rosana Pereda; ``Sanatorio Guemes''-Soledad Zarate; ``HIGA Evita’’-Ana Togneri; HIGA ``Dr. José Penna’’-María L. Benvenutti, Mabel Rizzo; HIGA ``Dr. Abraham F. Piñeyro’’ Monica Machain; ``HIGA Luisa C. de Gandulfo’’-Andrea Fascente; ``Hospital Municipal de Agudos’’ ``Dr. Leónidas Lucero’’-Laura Paniccia; ``HIGA Presidente Perón’’-María Adelaida Rosetti; HIGA ``Vicente López y Planes’’-Hebe Gullo, María Susana Commisso; Hospital Zonal General de Agudos ``Dr. Carlos Bocalandro’’-Carolina Baccino, Nory Cerda; Hospital Materno Infantil ``Dr. Victorio Tetamanti’’-Victoria Monzani, Laura Morvay; Hospital Municipal ``Dr. Bernardo Houssay’’-Micaela Sogga; Hospital Universitario Austral-Viviana Vilches; Hospital de Niños ``Sor María Ludovica’’-Cecilia Vescina; Hospital Municipal de Pediatría ``Dr. Federico Abete’’- Liliana Esteves; Hospital del Niño de San Justo-Johanna Perez, Liliana Meccia; HIGA ``Eva Perón’’-Marisa Almuzara; Hospital Municipal ``Ramón Santamarina’’-Monica Sparo; Hospital Municipal ``Dr. Federico Falcon’’-Gabriela Galán; Hospital Municipal ``Ostaciana V. Lavignolle’’-Roxana Depardo; ``HIGA Simplemente Evita’’-Maricel Garrone; Hospital Nacional ``Prof. Dr. Alejandro Posadas’’-Adriana Fernández Lausi, Graciela Priore; Hospital Privado de Comunidad-Monica Vallejo; Hospital Zonal General de Agudos ``Virgen del Carmen’’-Adriana Melo; Hospital Municipal ``Dr. Pedro Orellana’’-Cecilia Barrachia; Hospital Zonal General de Agudos ``Virgen del Carmen’’-Adriana Melo; Hospital ``Dr. Lucio Molas’’-Gladys Almada; Hospital ``Gobernador Centeno’’-Adriana Pereyra; Laboratorio Central de Salud Publica Catamarca-Daniela Carrizo; Hospital Interzonal de Niños ``Eva Perón’’-Patricia Valdez, Mariela Silvia Farfan; Hospital Pediátrico ``Dr. Avelino Castelán’’ Chaco-Leyla Guadalupe Gómez Capara; Mónica Graciela Sucin, Viviana Isabel Saito; Hospital 4 de Junio ``Dr. Ramón Carrillo’’-Norma Ester Cech; Hospital ``Dr. Julio C. Perrando’’ Laura Picoli, Mariana Carol Rey, Isabel Ana Marques; Hospital Regional ``Dr. Victor M. Sanguinetti’’-Chubut-Susana Ortiz; Laboratorio de la dirección de patología prevalente y epidemiología-Mario Flores; Red de Laboratorios-Diana Berry; Hospital Zonal de Trelew-Teresa M. Strella; Hospital Zonal de Esquel-Omar Daher; Hospital ``Dr. Guillermo Rawson’’ Córdoba-Ana Littvik; Hospital Regional ``Dr. Louis Pasteur’’-Claudia Amareto de Costabella; Clínica Privada Vélez Sársfield-Lidia Wolff de Jakob; Hospital Regional ``Domingo Funes’’-Lilia Norma Camisassa; Clínica Universitaria ``Reina Fabiola''-Marina Botiglieri; Hospital Infantil Municipal Córdoba-Liliana González; Hospital de Niños ``Santísima Trinidad’’-Patricia Montanaro; Hospital Pediátrico Del Niño Jesús-Paulo Cortez; Hospital ``Ángela Llano''-Ana María Pato; Hospital Pediátrico ``Juan Pablo II''-Adriana Wolfel; Hospital Materno Infantil ``San Roque’’ Entre Ríos-Lorena del Barco, Maria Silvia Diaz, Maria Eugenia de Torres; Hospital ``Delicia C. Masvernat’’-María Ofelia Moulins, Luis Otaegui, Norma Yoya; Hospital ``Dr. Lucio Molas’’-Gladys Almada; Hospital ``Gobernador Centeno''-Adriana Pereyra; Hospital de la Madre y el Niño-Nancy Comello, Silvana Vivaldo; Hospital Central-Nancy Noemí Pereyra; Hospital Regional ``Dr. Enrique Vera Barros''-La Rioja-Sonia Flores, Mónica Romanazi; Hospital de Niños ``Dr. Héctor Quintana''-Marcelo Toffoli, Gabriela Granados; Laboratorio Central de Salud Pública-Beatriz Resina; Hospital Pediátrico ``Dr. Humberto Notti’’-Laura Balbi, Alfredo Matile, Beatriz García; Hospital ``Dr. Teodoro J. Schestakow''-Ada Zanusso, Adriana Edith Acosta; Hospital Provincial de Pediatría ``Dr. Fernando Barreyro’’ Misiones-Martha Von Spetch, Sandra Grenon, Lorena Leguizamón; Hospital Escuela de Agudos ``Dr. Ramón Madariaga''-Viviana Villalba; Hospital Provincial ``Dr. Castro Rendón’’ Neuquén-Cristina Pérez; Red de Laboratorios-Evelin Oller; Hospital ``Dr. Horacio Heller''-Fernanda Bulgueroni; Hospital Zonal Bariloche ``Dr. Ramón Carrillo’’ Rio Negro-Néstor Blázquez, María Laura Álvarez; Hospital Área Cipolletti-Cristina Carranza, Mariela Roncallo; Hospital ``Francisco López Lima''-Gonzalo Crombas, Daniela Durani; Hospital ``A. Zatti''-Graciela Stafforini, María Gabriela Rivolier; Hospital ``Presidente Perón’’ Salta-Cristina Bono, Eloisa Aguirre; Hospital Público Materno Infantil-Ana Berejnoi; Programa Bioquímica de Salta-Jorgelina Mulki; Hospital ``San Vicente de Paul’’-Silvia Amador; Hospital del Milagro-Norma Sponton; Hospital ``Dr. Guillermo Rawson’’ San Juan-Marisa López,; Hospital ``Marcial Quiroga''-Hugo Castro; Policlínico Regional ``Juan Domingo Perón''-San Luis-Ema María Fernández; Hospital Privado San Luis-Hugo Rigo; Hospital Zonal de Caleta Olivia ``Pedro Tardivo’’ Santa Cruz-Josefina Villegas; Hospital Regional de Rio Gallegos-Mariel Borda, Alejandra Vargas, Wilma Krause; Hospital de Niños ``Dr. Víctor J. Vilela’’ Santa Fe-Andrea Badano, Adriana Ernst, Mariel Borges; Laboratorio Central de Salud Pública-Andrea Nepote, María Gilli; ``CEMAR''-María Inés Zamboni, Julieta Valles; Hospital ``Dr. José M. Cullen''-Emilce Méndez, Alicia Nagel; Hospital Español-Santa Fe-Noemí Borda; Hospital de Niños ``Dr. Orlando Alassia’’-Stella Virgolini, María Rosa Baroni; Hospital Regional ``Dr. Ramón Carrillo’’-Marciana Cragnolino Santiago del Estero; Hospital de Niños (CePSI) ``Eva Perón’’-María Elisa Pavón; Hospital Regional Ushuaia-Manuel Boutureira; Hospital Regional Río Grande-Marcela Vargas, Alejandra Guerra; Hospital del Niño Jesús-Tucumán-Ana María Villagra De Trejo, José Assa; Hospital de Clínicas ``Presidente Dr. Nicolás Avellaneda’’-María Fernández de Gandur; Laboratorio de Salud Pública-Norma Cudmani; ``Instituto Argentino de Diagnostico y Tratamiento''-Gabriela Boscaro; ``Sanatorio Sagrado Corazón''-Julián Fazio; ``Instituto Alexander Feliming''-M. Blanchery; ``Htal. Zonal Materno Infantil Argentina Diego''- Stella Maris Altamiranda; ``Htal. Juan A. Fernández''-Liliana Guelfand; ``Htal. Dr. Arturo Oñativia'' Ana Laura Mariñansky; ``Htal. Larcade''-Rech Sabrina; ``Htal. Narciso López'', Lanús-Jimena Zandonadi; ``Htal. E. Tornú''-Liliana Longo; ``Htal. San Juan de Dios-La Plata''- Andrea S. Pacha; ``Hospital Municipal de Tigre’’-Tolini Maria Cecilia; Htal. ``Blas l. Dubarry’’-Ana Gómez; ``Htal. Español, CABA''-Scolnik; ``Hospital Nuestra Señora de Luján''-Araceli Burella; ``Hospital Emilio Zerboni''-Sofia Murzicato; ``Clínica del Niño y la Familia, Quilmes''-Martínez Coleman Verónica,Galiñanes Sebastián; ``Hospital San José''-Zanotto M. Cecilia; ``Clínica Constituyentes''-Rosaura Taboada; ``Hospital Dr. T Álvarez, CABA''-Liliana Rodriguez; ``Fundación Hospitalaria''-Alejandra Lasont; ``Instituto Modelo de Cardiología SRL, Córdoba''-María Soledad Muñoz; ``Htal. Iturraspe, Santa Fe''-Maneiro Guillermina; ``Htal. Militar Central Cirujano Mayor Cosme Argerich''-Nora Gómez, Tte. Coronel Perret Sonia; Htal de Clinicas ``Jose de San Martin''-Carlos Vay; Hospital General de Agudos ``Dr. Ignacio Pirovano''-Claudia Garbaz; Hospital General de Agudos``Dr. Parmenio Piñero''-Daniela Ballester, Flavia Amalfa; Hospital Alemán-Liliana Fernandez Caniggia; Hospital General de Agudos ``Dr. Carlos.G. Durand''-Marta Flaibani; FLENI-Nora Orellana; Hospital General de Agudos ``Dalmacio Vélez Sarsfield’’-Silvana Manganello; Hospital ``Churruca-Visca’’-Iliana Martinez; HIGA ``Dr. Pedro Fiorito’’ Silvia Beatriz Fernández; HIGA ``Dr. Diego Paroissien’’-Maria R Cervelli, Hospital Zonal Especializado de Agudos y Crónicos ``Dr. A. Cetrangolo’’-Appendino Andrea, Laura Biglieri; Hospital Zonal Gdor. Domingo ``Mercante''-Sandra Bognanni; Hospital Municipal ``Dr. Enrique Sturiz''-Alejandra Sale; Hospital Interzonal General de Agudos ``Dr. Enrique Erril''-Victoria Ascúa.
Received: 4-1-2022
Accepted: 8-8-2022












 uBio
uBio