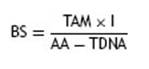Introduction
Meat is a valuable source of protein in the human diet, which makes it prone to high microbial contamination that can cause spoilage and foodborne infections in human beings2. The microorganisms most commonly present in meat and meat products are Salmonella spp., Escherichia coli, Bacil-lus spp., Clostridium spp., and Shigella spp.22. For this reason, the safety and quality of meat must be ensured.
Salmonellosis is among the main foodborne diseases affecting human beings globally. Since animals are the principal reservoirs of Salmonella spp., beef has been implicated as a vehicle of human infection in several outbreaks27. Shiga toxin-producing Escherichia coli (STEC) is a known enteric pathogen associated with foodborne illnesses, representing an important human health problem40. Ruminants are one of the main STEC reservoirs, and it has been recently shown that around 18.2% of all STEC reported cases worldwide were associated with beef40.
In abattoirs, carcass contamination with pathogens can occur through contact with the hide and contents of the digestive tract of slaughtered animals as well as through contact with contaminated workers’ hands, equipment or utensils10.
Argentine abattoirs are classified into exporter, federal transit and provincial transit according to their slaugh-ter capacity, the marketing area for meat and viscera, and the sanitary authority responsible for their control16. Hygiene and sanitation standards differ among the abattoir categories described above34. For instance, most provincial transit abattoirs do not apply Hazard Analysis Critical Control Point (HACCP), Standardized Sanitary Operational Procedures (SSOP) or any intervention procedure at the end of meat processing8.
In Argentina, Costa et al.16 reported the presence of E. coli O157:H7 (3.3%), non-O157 STEC (18.8%) and Salmonella spp. (5.5%) in 60 carcasses from three abattoirs with no HACCP plan in Buenos Aires province, before making improvements. In addition, in Tucumán, Perez Terrazzino33 et al. reported E. coli O157:H7 (0.46%) and non-O157 STEC (1.9%) in 274 carcasses from six abattoirs without HACCP plan.
This work focused on the comprehensive study of two provincial transit abattoirs without an HACCP plan from Tucumán (Argentina) by evaluating their sanitary status, including risk estimation and determination of the bacte-riological quality of carcass and environmental samples.
Materials and methods
The province of Tucumán (22 524 km2; 1 448188 inhabitants) has eight provincial transit abattoirs controlled by the Subsecretaría de Asuntos Agrarios y Alimentos. This study was conducted in two of these abattoirs with no HACCP plan, identified as abattoir I (26°49'26" S 65° 13'21" W) and abattoir II (27°3'15" S 65°24'12" W). Each abattoir was visited once a week for 20 consecutive weeks, during the operational and post-operational processes, in the period 2016-2018. Risk was estimated using two checklists. In addition, the bacteriological analysis of carcass and environmental sam-ples (platforms, saw machine, worker’s hands, knives, trays, sinks) was performed.
Risk estimationRisk was estimated using the two checklists described by Costa et al.16 with minimal modifications. The pre-operational checklist of this work was used as post-operational checklist (once work was finished and after complete cleaning of the abattoir) in the present work. Ten operational and 10 post-operational checklists were com-pleted alternately for 20 weeks in each abattoir. They were used to evaluate different variables associated with the building conditions of the establishment, the production line and good manufacturing practices (GMP), good hygiene prac-tices (GHP) and SSOP. Each variable was assigned a numerical value and grouped into six blocks. Each block was assigned a score depending on the importance of the sector in the risk of contamination. The four possible qualifications of abattoir conditions were acceptable (perfect), marginal (not ideal), unacceptable (not corresponding) or not applicable (could not be evaluated but did not influence process outcomes).
The final block score (BS) was obtained with a formula that included the sum of the total acceptable and marginal grades obtained (TAM) multiplied by the importance (I) assigned to each block, divided by the sum of all accep-table scores (AA) minus total grades referred to as ''does notapply’’ (TDNA)16.
The sum of all BS gave a final score of 100. Accordingly, risk was estimated ona 1-100scaleashigh (1-40), moderate (41-70) or low (71-100).
The operational checklist included the following blocks and scores: (1) pens (15.0); (2) slaughter area (25.0); (3) head and viscera area (10.0); (4) control points (15.0); (5) cool chambers (20.0); (6) offal area (15.0). The post-operational checklist included the following blocks and scores: (1) pens (15.0); (2) slaughter area (35.0); (3) cool chambers (10.0); (4) quartering (10.0); (5) offal area (20.0); (6) outdoors (10.0).
Sample collectionCarcass (n = 160) and environmental (n = 136) samples were obtained using a sterile sponge (Whirl-Pak Speci-Sponge, Nasco, USA) soaked in 10 ml of buffered peptone water (BPW) (Britania S.A., CABA, Argentina).
At each visit, carcass samples (n = 4) were taken during the operational (airing chambers) and post-operational (cool chambers) processes. Two samples from each carcass were taken. One was a combination of four carcass areas of 100 cm2 each (chest, neck, buttock and posterior lateral hock), which were swabbed for the count of indica-tor microorganisms35: E. coli/coliform and mesophilic. The other sample was obtained by swabbing the carcass entire surface (external and internal side) with another sterile sponge (Whirl-Pak) and used for the detection of Salmonella spp., E. coli O157:H7 and non-O157 STEC16.
In the case of environmental samples, different pools were obtained during each operational visit from platforms (n=4), saw machine (chest and half, n=2), workers’ hands (n=4) and knives (n = 4). During post-operational visits, pools of platform (n = 4), saw machine (chest and half, n = 2), tray (n = 4) and sink (n =4) samples were used for the detection of Salmonella spp., E. coli O157:H7 and non-O157 STEC. All samples were stored at 4 °C and immediately sent to the laboratory for analysis.
Bacteriological analysisCarcass samples were analyzed for indicator microorganisms with 3M Petrifilm™ E. coli/coliform count plates and mesophilic count plates (3MTM, Minnesota, USA). After the addition of 10 ml BPW (Britania S.A.), the bags with the carcass sampling sponges were homogenized and squeezed. Then, 1 ml of the sample was placed into each 3M Petrifilm™ plate, incubated and counted according to the manu-facturer’s specifications. Results were expressed as log CFU/cm2.
Escherichia coli O157:H7, non-O157 STEC and Salmonella spp. were detected and isolated from carcass and envi-ronment samples. Sponges were aseptically cut in half and 500ml BPW was added. They were then aseptically divided into two portions of 250 ml each to analyze the different bacteria.
The detection and isolation of E. coli O157:H7 and non-O157 STEC was performed according to USDA MLG 5C.0031, with some modifications. The rfbO157 gene and Shiga-toxin (síx) genes síxi and stx2 were screened by multiplex-polymerase chain reaction (PCR)28. Escherichia coli serotyp-ing was carried out using agglutination tests on a microplate, slide and/or tube containing specific antisera (Instituto Nacional de Producción de Biológicos [INPB] - ANLIS ''Dr. Carlos G. Malbrán’’, Argentina). Cultures that could not be resolved by serotyping were subjected to multiplex PCR to determine the gene encoding somatic antigen (O)1.
Isolation of Salmonella spp. was carried out according to ISO 65 79-1:201 724 and serotyping was performed according to the White-Kauffmann-Le Minor scheme by slide (O antigen) and tube (H antigen) agglutination, using specific antisera (INPB - ANLIS).
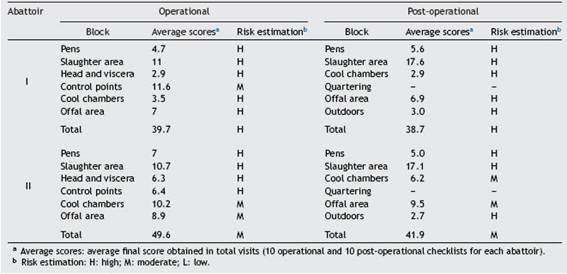
Table 1: Risk estimation obtained in the operational and post-operational visit of abattoirs I and II.
Statistical analysesThe microbiological quality of carcasses (mesophilic microorganisms, coliforms and E. coli counts) from both abattoirs was evaluated using the Student’s í-test for inde-pendent variables. Enumeration data were log-transformed before performing the analysis of variance. All statistical analyses were performed using IBM® SPSS® version 24. Sig-nificance was set at p <0.05.
Results
Risk estimation
Risk was high to moderate in the two abattoirs and in both operational and post-operational processes, with predomi-nance of high risk.
In abattoir I, the results obtained with the operational checklist showed that risk was high and moderate (range, 33-49; 10 visits), with an average of 39.7 (high) in the pens, slaughter, head and viscera, cool chamber and offal sec-tors. Only control points were rated as having moderate risk. Likewise, the risk values obtained with the post-operational checklist were also classified as high and moderate (range, 26-51; 10 visits), and 38.7 in average (high risk). Such high risk was detected in all sectors (pens, slaughter area, cool chambers, offal area and outdoors) during at least one visit. The average scores of each block are shown in Table 1.
In abattoir II, the operational checklist rendered moderate risk results in the cool chambers and offal area (range, 46-53; 10 visits), with an average of 49.6. However, the risk was high in the pens, slaughter area, head and viscera area and control points. Concerning the post-operational checklist, the values obtained were in the range of 33-48 (10 visits) and also classified as high (pens, slaughter area and outdoors in at least one visit) and moderate (cool chambers and offal area) risk. In this case, the average corresponded to moderate risk (41.9). The average scores of each block are shown in Table 1.
The main deviations from HACCP observed in both abattoirs were (a) deficient building conditions: pen infrastructure, slaughter area, carcass and offal cool chambers (walls, floors and ceilings, platforms, lighting and ventila-tion, among others); (b) inadequate workflow, including lack of workers; (c) lack of sectorization of changing rooms and bathrooms; (d) lack of implementation of SSOP, particularly during the post-operational process, and (e) no food safety training of workers.
Bacteriological analysisThe counts of mesophilic microorganisms, coliforms and E. coli from abattoirs I and II did not differ significantly. In Argentina, only E. coli is included as microbiological criteria for microorganism counts on carcass surfaces (limit, <0.7 log CFU/cm2)35. In abattoir I, counts were as follows (log CFU/cm2): mesophilic, 3.1 ±0.14; coliform, 0.56±0.01; E. coli, 0.49±0.03. According to local regulations (SENASA, 2008), 32 samples (40%) did not comply with the microbiological criteria for E. coli. Likewise, abattoir II showed the following counts (log CFU/cm2): mesophilic, 2.6 ±0.42; coliform, 0.47±0.05; E. coli, 0.41 ±0.07. Accordingly, 13 samples (16.2%) did not meet the microbiological criteria for E. coli.
The síx genes were detected in 39 (24.4%) carcass and 42 (30.9%) environmental samples. While all samples (carcass and environmental) and sampling times (opera-tional and post-operational) from both abattoirs had at least one síx-positive sample, E. coli O157:H7 was not isolated from any of them. On the other hand, STEC was isolated from three (1.9%) carcass and five (3.7%) environmental samples, as follows: in abattoir I, it was isolated from two carcasses (post-operational) and one environmental (operational) sample. The STEC isolates were characterized as E. coli O8:H7/síXi and O116:H49/síx2 in carcass and O136:H40/síx2 in platform samples. In abattoir II, STEC was isolated from one carcass (post-operational) and four environmental (operational and post-operational) samples, and the isolates were characterized as E. coli O113:H21/síx2 (carcass), O116:H49/síx2 and O7:H7/síx2 (trays), O8:H16/síx2 (platforms) and O178:H19/síx2 (workers’ hands).

Table 2: STEC detection and isolation and Salmonella spp. isolation from carcass and environmental samples from each abattoir during operational and post-operational processes.
Salmonella spp. was isolated from six (7.5%) carcass and 10 (7.3%) environmental samples. In abattoir I, Salmonella isolates (n = 3) came from the post-operational process. The serovars identified were S. Cerro in two carcass samples and
S. Corvallisin one tray sample. In abattoir II, Salmonella was isolated from carcass and environmental samples (n = 13) in both the operational and post-operational processes. Serovars were identified as S. Cerro (n=4, platform, saw machine, knives and worker’s hands), S. Corvallis (n = 3, carcass), S. Havana (n = 3, two from workers’ hands and one from knife) and S. Agona (n = 3, carcass, saw machine and tray).
Results of STEC detection and isolation and Salmonella spp. isolation in carcass and environmental samples are shown in Table 2. Sources of Salmonella spp. isolation and STEC detection and isolation from environmental samples are detailed in Table 3.
Discussion
In this work, the comprehensive study of two provincial tran-sit abattoirs with no HACCP plan from Tucumán, Argentina, was carried out through risk estimation and sampling of car-casses and the environment.
Around 30.0% of the beef produced in Argentina is for export, and the remaining 70.0% is destined for domestic consumption8. Exporting companies produce premium qual-ity products which comply with the high levels of demand and the required high quality standards. In the domestic market, however, these standards depend on local demands and local-state control. Like in other countries, this may occasionally result in multiple abattoir hygiene and sani-tation standards17,25,34. It has been shown that consuming meat from abattoirs without HACCP increases the risk of contracting diseases such as hemolytic uremic syndrome8. In this sense, 16% of the Argentinian bovine slaughter comes from provincial abattoirs with no HACCP plan16.
In a previous study conducted in Argentinian abattoirs, risk estimation checklists and microbiological analyses were useful to identify relevant deviations from HACCP16. In this study, the same model was applied in the two abattoirs evaluated during the operational and post-operational processes. The results obtained indicated the predominance of high risk of contamination in both abattoirs and high-lighted the lack of control along the production line and after the sanitation process, two important points where the microbiological quality of meat should be ensured13. In addition, the existence of major failures was evidenced, not only in buildings (lack of sanitary filters, divisions between dirty and clean areas, enclosures) and processes (lack of GMP, GHP, SSOP, HACCP), but also in the lack of safety training of workers. Similar problems have been recently reported in abattoirs from Ethiopia and Namibia6,21,32. In this regard, three Argentinian abattoirs without HACCP managed to reduce the risk of contamination from high to moderate through the implementation of improvement actions16. Based on the results obtained in the current study, it would be possible to implement an improvement plan in each abat-toir from Tucumán jointly with the local health authorities, including the training of workers.
The mesophilic counts reported here were similar to those obtained in Buenos Aires (BA) abattoirs without HACCP16, but lower by more than 1 log CFU/cm2 in abattoir II compared with BA results. Coliform counts were in the same order as those obtained in two abattoirs from BA after applying improvement actions16, while E. coli counts were higher. However, counts in abattoirs without HACCP from Argentina were lower (1-4log CFU/cm2) than those from Pakistan and Ethiopia2,6. On the other hand, mesophilic, coliform and E. coli counts were almost 2 log CFU/cm2 higher in Argentine abattoirs without HACCP than in exporting abat-toirs applying an HACCP plan36, supporting the importance of applying a quality control system.
High counts can be indicative of the possible presence of pathogens29, although low counts do not ensure the total absence of potentially pathogenic bacteria such as STEC. In this study, the detection of stx from carcass and envi-ronmental samples was low (24.4 and 30.9%, respectively). In BA abattoirs, stx was detected in 97.2% of carcass and 76.1% of environmental samples15. Differences could be due to the stx screening technique used, i.e., real-time PCR in Costa15 and conventional multiplex PCR in this work, whose detection limit is 1000 times higher28. In addition, the PCR currently used did not include an internal amplification control, so that the detection of stx could have been underestimated by false negative results23. On the other hand, the limited success to isolate non-O157 STEC should be highlighted. In BA abattoirs, STEC were isolated from 18.8% carcass and 6.1% environmental samples in the initial stage16, while in the current work this was only possible in 1.9% carcass and 3.7% environmental samples. Factors such as high levels of background bacteria, volume of samples plated, amount of plates necessary to achieve STEC isolates, number of colonies selected per plate, and laboratory per-sonnel experience probably impaired the accurate isolation of STEC4,9,17,26.
The STEC serotypes O8:H16, O113:H21 and O178:H19iso-lated in this study have been previously associated with unique cases of hemolytic uremic syndrome20. However, there are no reports of disease cases caused by these serotypes in Tucumán. Serotypes O113:H21 and O178:H19 were also reported in carcass, viscera and environmental samples from BA provincial abattoirs16. The other STEC serotypes isolated in this study were previously isolated in cattle from Argentina, reaffirming that cattle are a reser-voir for a wide variety of STEC strains11,18,19,38. Meanwhile, E. coli O157:H7 was not isolated, probably due to the high levels of background flora detected in the samples and the low capacity to compete for this E. coli serotype37,39.
A wide variety of reports account for the pres-ence of Salmonella spp. both in carcasses and abattoir environments, highlighting the possibility of cross-contamination1,26,31. The prevalence of Salmonella spp. in carcasses differs among countries, ranging from 0.1% in Mid-western USA5 to 18.0% in Mexico30 and 9.9% in Ethiopia6. In the present study, the prevalence of Salmonella spp. was 3.8% in carcass and 7.4% in environmental samples from both abattoirs. Such prevalence was higher than that described by Costa et al.16 in abattoirs from BA (Argentina), where Salmonella spp. was not detected in carcasses, being its prevalence 4.1% in environmental samples after the imple-mentation of improvement actions.
The Salmonella serovars isolated here were S. Cerro, S. Corvallis, S. Agona and S. Havana. Even though all of them had already been reported in Argentina in different types of samples14,16, none of them corresponded to the most prevalent serovars in human diseases in Argentina12. Con-sidering that Salmonella can be transmitted directly and indirectly7, its presence in any food compromises food safety and is indicative of poor hygiene practices such as regular and effective handwashing after visiting the toilet or mixing meat with intestinal contents during carcass dressing.
Non-compliance of companies with regulations and lack of adequate state-control has been defined as ''organized irresponsibility’’3. In Argentina, with the exception of exporting companies, provincial bovine abattoirs do not receive economic stimulus for applying high hygiene stan-dards. This does not promote fair competition, considering that abattoirs applying good hygiene practices and selling their production in the local market do not receive an appro-priate economic reward either.
Conclusions
Based on the results of this work, it was possible to corroborate that checklists together with sampling to validate their application were useful tools to know the sanitary condition of two abattoirs, mainly classified as having high risk. Carcasses had high counts of indicators (mesophilic bacteria, coliforms and E. coli) as well as STEC and Salmonella, which were found on the products and the environment at two specific times of the production process, operational and post-operational. These results will allow to plan improvements in Tucumán abattoirs aimed at solving the problems identified through risk estimation and microbiological analysis. Still, the need to work jointly with the sanitary authority in search of a single sanitary standard for Argentina based on the implementation of HACCP in all abattoirs, regardless of category, remains unaddressed.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.Conflict of interest
The authors declare that they have no conflicts of interest.
Acknowledgement
We thank A. Di Maggio for correcting and editing the manuscript.
Received 26 November 2021
accepted 16 November 2022












 uBio
uBio