Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Latin American applied research
versão impressa ISSN 0327-0793
Lat. Am. appl. res. v.32 n.2 Bahía Blanca abr./jun. 2002
Synthesis of chemically induced separation sequences using fuzzy heuristic based system
L. A. Cisternas†, Ch. J. Guerrero† and J. A. Melo‡
† Departamento de Ingeniería Química, Universidad de Antofagasta, Casilla 170, Antofagasta, Chile
lcisternas@uantof.cl
‡ Departamento de Ingeniería en Sistemas, Universidad de Antofagasta, Casilla 170, Antofagasta, Chile
Abstract — Chemical induced separation is used in a number of instances to accomplish difficult separations; however, there is a lack of information in the literature. In the present work, a fuzzy heuristic system for separation sequence synthesis using chemical induced separation is constructed. The construction of the present rule based system is based on the previous work of Huang and Fan (1990) in which knowledge-based system has been applied to physically induced separation sequence synthesis. Although the methodology proposed here, involves the use of declarative and procedural knowledge the emphasis is done on selection and sequence of chemical induced separation. A simple example is included to illustrate the procedure.
Keywords: Fuzzy Heuristic; Separation; Process Synthesis; chemical induced separation.
I. INTRODUCTION
Chemically induced separation is a common technique used for difficult separation. However, the synthesis of such systems has not received enough attention, and only few articles are available in the literature. Berthouex and Rudd (1977) developed a conceptual design, through an inductive reasoning, for the processing of red mud using chemically induced separation. Othmer and Nowak (1972) showed that the chemical affinities of halogens can be used in order to carry out difficult separations. Cisternas (1999) proposed a hierarchical morphology analysis to develop conceptual design for chemically induced separation.
On the other hand, there are many works on physically induced separation synthesis using knowledge-based systems and mathematical programming approaches. Examples of these works are: Hendry and Hughes (1972), Floudas and Paules (1988), Stephanopoulos and Westerberg (1976), Huang and Fan (1988), Qian and Lien (1994), and Wang et al. (1998).
Consider the following two examples as illustration of chemically induced separation:
1) The extraction of copper from a concentrated copper sulfide minerals by pyrometallurgical techniques. The extraction consists of roasting, matte smelting, and converting to blister copper. Roasting, an optional step, consists of partially oxidizing the sulphides and of partially eliminating sulphur from them as SO2. The iron oxides and sulphates are subsequently eliminated during smelting as slag. Matte smelting consists of melting concentrates or partially roasted concentrates to produce two immiscible liquid phases consisting of oxides (slag) and sulphide (Cu2S-FeS matte). The presence of FeS in the matte tends to sulphidize all the copper present in the charge. This reaction also allows the partial oxidation of iron, which is eliminated in the slag. The third stage, converting of copper matte, consists of blowing air through the mass of molten copper matte with the purpose of removing iron, sulphur and other impurities. The converting process takes places in two steps: a) slag forming stage in which FeS is oxidized, and b) the copper-making stage in which the copper sulphide is oxidized.
2) Another example is the separation system in the production of lithium carbonate from natural brines. In this process, sodium carbonate is used to precipitate magnesium carbonate, calcium carbonate, and lithium carbonate in several steps to separate Mg+2, Ca+2, and Li+ from an aqueous solution.
Chemically induced separation is usually considered when: (1) the components of a mixture have similar properties, so that a physical separation is unfeasible or difficult, and (2) the valuable components are present in small amounts.
In this work, a fuzzy heuristic based system for the synthesis of chemically induced separation sequences is presented. The aim is not to develop a complete rule based system, but to prove that this kind of synthesis problem can be solved using knowledge based systems.
II. PROBLEM SPECIFICATION
This problem can be defined as follows "Given a mixture of known composition, synthesize a sequence of chemical reactions and physical treatment that allows the separation of their species at minimum venture cost." The synthesis for separation involves three major steps: selection of a chemical transformation that allow the separation, determination of the order in which the various separations should be performed, and evolution of the initial scheme.
Separation schemes presented in this work only involve single feed stream, pure product streams, a single chemical induced separation method, sharp separators, and no heat integration. Therefore, the problem is kept simple in order to maintain the attention on the use of Gibbs free energy in the heuristic rules employed.
III. KNOWLEDGE REPRESENTATION STRATEGY
A knowledge base for complex separation scheme synthesis, as for other complex applications, must contain both declarative and procedural knowledge. In the present approach, knowledge of both types is represented in the form of objects. The representation utilized is similar to the one used by Huang and Fan (1990), and therefore only a brief description is given here.
A. Declarative Knowledge
Knowledge manifesting itself as a set of facts is accepted to be declarative. In this work, at least four groups of declarative knowledge have been identified and deemed useful for the synthesis of chemically induced separation scheme. They include information concerning the results of previously solved problems , the properties of chemicals, feasible chemical reactions, and the status of a current solution. The general data structure is depicted in Fig. 1.
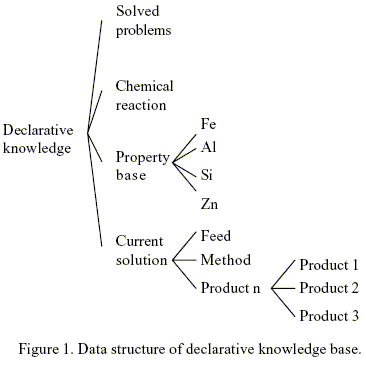
The general data structure for previously solved problems contain the list of compounds to be separate by chemical transformation, a list of products, and the chemical transformation used. When the list of compounds of a new problem match precisely those of an entry in the known-case database, the chemical transformation of that entry is retrieved and employed for the new problem.
The chemical and physical properties of the compounds are included in the class Property_Base in Fig. 1.This information is important when the specifications of a new separation case don`t match those of a known case, decisions concerning which chemical transformation to select and, subsequently, what arrangement should be made for each separator are usually made in the light of the chemical and physical properties of the chemical to be separated and potential products. In this work Gibbs free energy of different chemical transformation are used to select the appropriate separation method. Their values are temperature dependent, and can be represented with the name, the chemical transformation, the chemical compound formed with the chemical transformation, the temperature, and the value of the Gibbs free energy of the chemical transformation. Also physical properties as boiling points and melting points are needed to analyze the feasibility of reactant/product separation in the reactor. The database in the approach of this work is not intended to encompass all properties values. Nevertheless, the data structure of the database is sufficiently flexible so as to accommodate more properties and their values whenever needed.
The Gibbs free energy is used to determine the relative affinities of the reactants as boiling point is used to determine relative volativity. The more affine an element the less is the value of the standard free energy change of reaction. As Gibbs free energy is a function of temperature, the relative affinity is temperature dependent.
The class Current_Solution contains the Feed, ProductN, and the Method (chemical transformation) as it is shown in Fig. 1. This class is used to represent the status of a current solution.
This work uses a reaction data-base made of known reaction, which are already known or proven experimentally to proceed at reasonable rates under specific conditions, for the representation of chemical reactions. The reactions are classified by the degree of truth value of the following linguistic terms "the reaction is appropriate for industrial uses." The judgment on a chemical reaction can be made by experts. The criteria can include 1. kinetic information (the reaction is violent, rapid, vigorous, low, etc.), 2. experience on the use of the reaction (the reaction is: an excellent method, used industrially, used in laboratory, etc.), 3. operation conditions (the reaction: occurs at low/high temperature, occurs catalyzed, is exothermic/endothermic, etc.), 4. safety and environmental issues.The class Chemical_Reaction contains a list of chemical reactions with its degree of truth.
B. Procedural Knowledge
In this work procedural knowledge is represented with heuristic rules. These rules, which are given in Table 1, are classified into three groups: selection of the chemical transformation, synthesis of an initial separation scheme, and evolution of the initial scheme.
Table 1. Heuristic rules employed in the present work for separation scheme synthesis
The rules (procedural knowledge) in the present approach are represented in the form of objects as mentioned earlier. Figure 2 illustrates the representation. The rules are organized into three classes (sets) namely Selection Rules, Sequence Rules, and Evolution Rules. Each member rule in a rule set is characterized by its name and the values stored in the five slots, including Weight, Antecedents, Consequences.
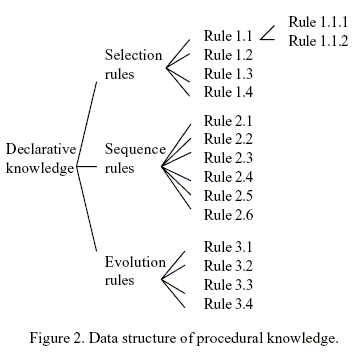
Slot Weight keeps the associated weight of each rule. The weights of individual rules are tabulated in Table 2. Those values are assigned subjectively after the relative importance of the rules is evaluated in the light of the comprehensive literature review (Huang and Fan, 1988); they represent the domain experts' general belief in weighing and balancing the rules and are independent of the separation method.
Table 2. Initial weights and highlights of the algorithms to calculate the truth value of the antecedents of each rule.
Slot Antecedents holds an algorithm to calculate the truth value of each rule; some highlights of the algorithms are found in Table 2. Considering Rule 1.1 as an example, the degree of truth of "Gibbs free energy vary widely" can be determined by the membership function,
| | (1) |
If a rule is selected the action to be taken is hold in the slot Consequences.
Rules 1.1, 1.2 and 1.3 can be applied at different temperatures as it is shown in Fig. 2 as rules 1.1.1 and 1.1.2. In these rules the claim "physical separation between products and reactants is easy" is evaluated by checking if the Min P(reactants) > Max P(products) or Max P(reactants) < Min P(products), where P is a physical property as melting point or boiling point.
Most of the rules don`t need further explanation because they are similar to those used in physical separation. In rules 2.2 to 2.6 the linguistic "adjacent physical properties of products vary widely" is used because if a mixture of converted reactants is formed, they need to be separated by physical separation.
Each linguistic term is quantified using fuzzy sets. The linguistic term is represented over the real number domain [0,1] by membership functions µA(x), whereµA(x)=1 means that x is definitely a member of A, µA(x)=0 means that x is definitely not a member of A, and intermediate values denote partial membership of A.
The assignment of weights to each rule enables us to discharge the rules in parallel. This ensures that each rule has an opportunity to participate in decision making commensurate with its weighted truth value; this prevents an under domination of any rule.
IV. EXAMPLE
A simple problem will be analyzed to illustrate the procedure. We will search for feasible flow sheets to separate a mixture of 37 percent aluminum oxide (Al2O3), 56 percent iron (III) oxide (Fe2O3), 5 percent zinc oxide (ZnO), and 2 percent silicon dioxide (SiO2).
The system commences by acquiring the name of the components in the feed and potential product compounds. In the quest for a separation method at the first level of synthesis, the system either resorts to its own (known-case and property) databases or lets the user specify the method. The choice is up to the user through manipulation of a menu. In the default mode, the system searches through the known-case databases first. A separation method will be recommended if it's found fitting or appropriate for the input specified. This is followed by a search in the property database for chemical transformation. If no method is identified in the first database, the system will still proceed with the search in the second. This is to discover a Gibbs free energy for chemical transformations whose values vary significantly among the specified components and that physical separation is possible in the reactor (reactants and products have different stable phase). If such a property is determined, a separation method will be selected based on the first set of knowledge sources, i.e., Rules 1.1 to 1.4 in Table 1. If no complete information is obtained after searching through both databases, the user will be prompted to specify a method, or certain separation factors, or both, depending on the missing information. The procedure is summarized in Table 3. From Table 3 is clear that the chemical transformation selected is chlorination at 900ºC.
Table 3. Evaluation of selection rules
The system engages in the second level of synthesis after a chemical transformation type is specified. Separators will be generated to form a separation train at this level. The decision is made within the discretion of the second set of knowledge sources (Rules 2.1 to 2.6 in Table 1). It's assumed that none of the components is corrosive. The most feasible separation is determined by the rule with the highest truth value. Thus, the overall truth value for all knowledge source is given in Table 4.
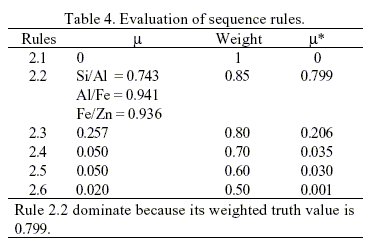
Knowledge source 2, i.e., Rule 2.2. clearly dominates at this step because its weighted truth value is 0.799, the greatest among the 6 knowledge sources. This mean to separate between Al/Fe compounds. This gives rise to the creation of the first separator illustrated in Fig. 3. The synthesis continues by repeating the steps described so far with a new set of source streams consisting of the unprocessed streams left from the previous step. An initial separation scheme has been obtained after two iterations of the procedure described above. The result is depicted in Fig. 3. The mixture of ZnCl2 - FeCl3 need further separation. The separation of this mixture is not included in this work because this kind of problem as been studied previously. However, the separation of ZnCl2 - FeCl3 mixture is possible because the linguistic "adjacent physical properties of products vary widely" as been included in rules 2.2 to 2.6.
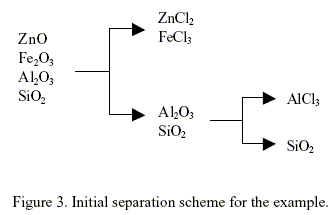
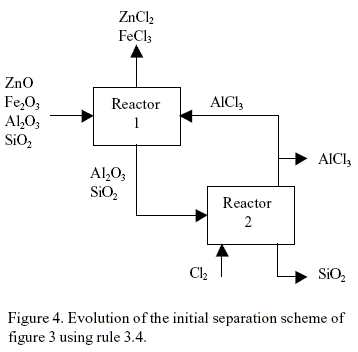
The application of rule 3.4 can be explained as follows. If a product of any reactor is less affine that a reactant of another reactor then this product can be used as a raw material in the last reactor. In this way the introduction of a new components or chemicals is avoided. For example, if rule 3.4 is applied to the initial separation scheme of Fig. 3, the flow sheet shown in Fig. 4 is obtained. The chemical reaction in reactor 2 is unfeasible (∆G > 10 kcal/mol). Using rule 3.3, the system can find a chemical reaction, if any is available in the data base, that can replace the unfeasible reaction of reactor 2.
V. DISCUSSION AND CONCLUSION
In this work the emphasis was done on selection and sequence, two crucial steps in generating a quality final separation scheme. Although the other level of analysis, namely, flow sheet evolution, is not fully demonstrated, their roles as integrated parts of a complete system should not be overlooked. The significance of the present work lies in the application of knowledge-based system to the synthesis of chemical induced separation sequences.
ACKNOWLEDGEMENT
The authors would like to acknowledge the financial support of the CONICYT for a grant (FONDECYT 1961270) in support of work of which this forms a part.
REFERENCES
1. Berthouex P.M. and D.F. Rudd, Strategy of Pollution Control, Wiley (1977).
2. Cisternas L.A., "On the synthesis of inorganic chemical and metallurgical processes. Review and extension", Mineral Engineering 12(1), 15-42 (1999).
3. Floudas, C. A. and G.E. Paules, "A mixed-integer nonlinear programming formulation for the synthesis of heat-integrated distillation sequence", Comput. Chem. Engg. 12, 531-546 (1988).
4. Huang, Y. W. and L.T. Fan, "Fuzzy logic rule based system for separation sequence synthesis: an object-oriented approach", Comput. Chem. Engg. 12(6), 601-607 (1988).
5. Hendry, J. E. and R.R. Hughes, "Generating separation process flowsheets", Chem. Engg. Prog. 68,. 71-76 (1972).
6. Othmer, D.F. and R. Nowak, "Halogen affinities-a new ordering of metals to accomplish difficult separations", AIChE J. 18 (1), 217-220 (1972).
7. Qian Y. and K.M. Lien, "Application of a fuzzy match inference strategy in synthesis of separation systems", Can. J. Chem. Eng. 72, 711-721 (1994).
8. Stephanopoulos, G. and A.W. Westerberg, "Studies in process synthesis—11. Evolutionary synthesis of optimal process flow sheets", Chem. Engg. Sci. 31, 195-204 (1976).
9. Wang K., Y. Qian, Y. Yuan and P. Yao, "Synthesis and optimization of heat integrated distillation systems using an improved genetic algorithm", Comp. Chem. Engng. 23(15), 125-136 (1998). [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ]
Received: November 14, 2000.
Accepted for publication: September 13, 2001.
Recommended by Subject Editor P. Toledo.














